News & Media
2021
Home / News & Media / 2021
2021
NEWS & MEDIA:

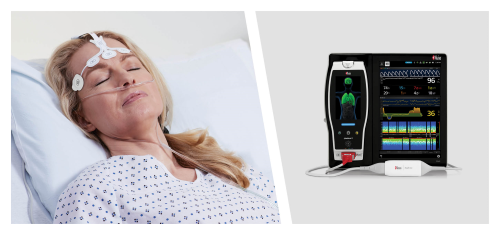
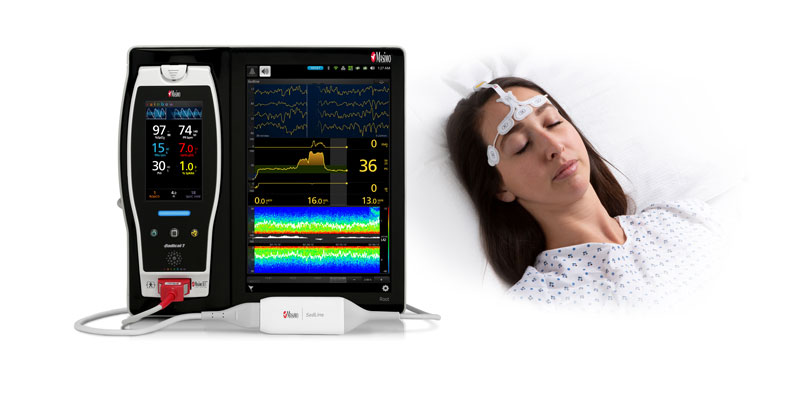
New Study Suggests That Masimo SedLine® Brain Function Monitoring May Aid Early Identification of Patients at Risk of Developing Postoperative Delirium
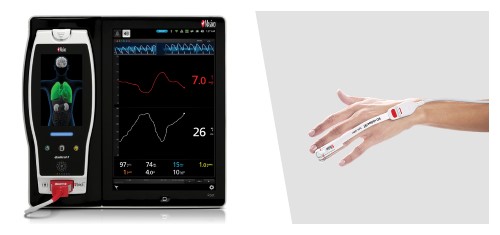
Neuchatel, Switzerland - December 13, 2021 - Masimo (NASDAQ: MASI) today announced the findings of a prospective study published in Anesthesia & Analgesia in which Dr. Claudia Spies and colleagues investigated the relationship between parameters derived from electroencephalogram (EEG) spectra, measured using Masimo SedLine® Brain Function Monitoring, and postoperative delirium (POD) in older patients undergoing elective surgery. The researchers found that the incidence of POD correlated with several spectral dynamics, in particular spectral edge frequency (SEF), suggesting that such EEG-based markers may help in early identification of patients at risk of developing POD.1
POD is a frequent complication in geriatric patients, often associated with worse short- and long-term outcomes and long-term cognitive dysfunction. Noting that the incidence of POD is associated with prolonged EEG burst suppression during general anesthesia, the researchers sought to investigate whether specific preoperative, preexisting EEG signatures might be related to a higher risk of developing POD.
The investigators enrolled 237 patients ≥ 65 years scheduled for elective surgery of at least 60 minutes at the Charité-Universitätsmedizin Berlin (Campus Virchow Klinikum and Campus Mitte) between November 2014 and December 2016. Using Masimo Root® with SedLine, frontal EEGs were recorded from before induction of anesthesia until return of consciousness. The researchers used the SedLine data to analyze a variety of EEG-derived parameters, including SEF (the frequency below which 95% of the power in the EEG is located), Patient State Index (a processed EEG parameter related to the effect of anesthetic agents), and duration of burst suppression, and also performed multitaper spectral analyses to calculate overall frontal power spectra across various frequency bands. Screening for POD was performed twice every day until the seventh day after surgery (or until hospital discharge) based on a variety of standard criteria, including the Nursing Delirium Screening Scale and Confusion Assessment Method. Patients with one or more positive screenings were classed as POD patients, and the remaining ones as NoPOD patients.
Of the 237 patients, 41 (17%) developed POD. The researchers found that two aspects of the preoperative EEG of POD patients was associated with lower values: SEF (POD group: 13.1 ± 4.6 Hz; NoPOD group: 17.4 ± 6.9 Hz; p = 0.002) and γ-band power (POD: -24.33 ± 2.8 dB; NoPOD: -17.9 ± 4.81 dB). Postinduction absolute α-band power was also significantly lower: POD: -7.37 ± 4.52 dB; NoPOD: -5 ± 5.03 dB. In POD patients, the ratio of preoperative to postinduction SEF was ~1; in NoPOD patients it was > 1, indicative of a slowing EEG with loss of consciousness. Finally, POD was independently associated with preoperative SEF (p = 0.025, odds ratios = 0.892, 95% CI 0.808 – 0.986), preoperative γ-band power (p = 0.029, OR = 0.568, 95% CI 0.342 – 0.944) and SEF ratio (p = 0.009, OR = 0.108, 95% CI (0.021 – 0.568).
The researchers concluded, "Lower preoperative SEF, absence of slowing in EEG while transitioning from preoperative state to unconscious state, and lower EEG power in relevant frequency bands in both these states are related to POD development. These findings may suggest an underlying pathophysiology and might be used as EEG-based marker for early identification of patients at risk to develop POD."
The authors also noted, "Preoperative spectral EEG signatures and reduced EEG dynamics at loss of consciousness are associated with the development of POD in older patients, where changes in EEG signatures are most likely related to reduced GABA-ergic neuronal activation in POD patients. These findings can be described as predisposing EEG factors for POD, which might be used as a potential EEG-based marker for early identification of patients at risk to develop POD."
David Drover, MD, Professor of Anesthesiology at Stanford Health Care, commented, "This study not only further supports existing knowledge but expands our understanding of how brain function monitoring can help clinicians improve postoperative outcomes in the elderly patient."
Postoperative delirium is an acute state of mental confusion characterized by alterations in attention, consciousness, and disorganized thinking. A common and serious complication, POD afflicts up to 60% of patients after major surgery,2-5 is most common in the elderly,2-5 and occurs in up to 91% of the critically ill.6 POD is associated both with worse short- and long-term outcomes and higher costs,3,6-9 and numerous medical bodies – including the American Society of Anesthesiologists (ASA), the United Kingdom National Institute for Health and Care Excellence, the American Geriatric Society, and the American College of Surgeons – have made the prevention of POD a public health priority.10-13 The ASA's Brain Health Initiative, dedicated to minimizing the impact of preexisting cognitive deficits and optimizing the cognitive recovery and perioperative experience for adults 65 years and older undergoing surgery, describes POD as a "major public health issue."14 The incidence of POD has been associated both with preoperative vulnerabilities and – of key importance to studies such as this – the cumulative duration of intraoperative EEG burst suppression. As numerous studies have found, processed EEG monitoring during surgery, by helping clinicians minimize the duration of burst suppression, may lower the rate of POD.15-19
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.20 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,21 improve CCHD screening in newborns,22 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.23-26 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,27 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.28 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Koch S, Windmann V Chakravarty S, Kruppa J, Yürek F, Brown E, Winterer G, Spies C. Perioperative Electroencephalogram Spectral Dynamics Related to Postoperative Delirium in Older Patients. Anesth Analg. Dec 2021. 13(6):1598-1607. DOI: 10.1213/ANE.000000000000005668.
- Lipowski ZL. Delirium in the elderly patient. N Engl J Med. (1989) 320:578–82. doi: 10.1056/NEJM198903023200907.
- Khadka J, McAlinden C, Pesudovs K. Cognitive trajectories after postoperative delirium. N Engl J Med. (2012) 367:30–9. doi: 10.1056/NEJMoa1112923.
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. (2014) 383:911–22. doi: 10.1016/S0140-6736(13)60688-1.
- Bin Abd Razak HR, YungWY. Postoperative delirium in patients undergoingtotal joint arthroplasty: a systematic review. J Arthroplasty. (2015) 30:1414–7. doi: 10.1016/j.arth.2015.03.012.
- Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. (2015) 350:h2538. doi: 10.1136/bmj.h2538.
- Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. (1994) 97:278–88. doi: 10.1016/0002-9343(94)90011-6.
- Crocker E, Beggs T, Hassan A, Denault A, Lamarche Y, Bagshaw S, et al. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thoracic Surg. (2016) 102:1391–9. doi: 10.1016/j.athoracsur.2016.04.071.
- Mashour GA, Woodrum DT, Avidan MS. Neurological complications of surgery and anaesthesia. Br J Anaesthesia. (2015) 114:194–203. doi: 10.1093/bja/aeu296.
- American Society of Anesthesiologists. Perioperative Brain Health Initiative Website. (2018). Available online at: https://www.asahq.org/brainhealthinitiative (accessed September 16, 2018).
- Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am College Surgeons. (2016) 222:930– 47. doi: 10.1016/j.jamcollsurg.2015.12.026.
- O’Mahony R, Murthy L, Akunne A, Young J. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Internal Med. (2011) 154:746–51. doi: 10.7326/0003-4819-154-11-201106070-00006.
- National Institute for Health and Care Excellence. Delirium in Adults. London: National Institute for Health and Care Excellence (2014).
- Brain Health. ASA. https://www.asahq.org/in-the-spotlight/brain-health. Accessed 13 Nov 2021.
- Tang CJ, Jin Z, Sands LP, Pleasants D, Tabatabai S, Hong Y, Leung JM. ADAPT-2: A Randomized Clinical Trial to Reduce Intraoperative EEG Suppression in Older Surgical Patients Undergoing Major Noncardiac Surgery. Anesth Analg 2020; 131(4):1228-1236.
- Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesthesia. (2013) 110:98–105. doi: 10.1093/bja/aet055.
- MacKenzie KK, Britt-Spells AM, Sands LP, Leung JM. Processed electroencephalogram monitoring and postoperative delirium: a systematic review and meta-analysis. Anesthesiology. (2018)129:417–27. doi: 10.1097/ALN.0000000000002323.
- Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. (2010) 85:18–26. doi: 10.4065/mcp.2009.0469.
- Whitlock EL, Torres BA, Lin N, Helsten DL, Nadelson MR, Mashour GA, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. (2014) 118:809–17. doi: 10.1213/ANE.000000000000002.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root and SedLine; risks that the researchers' conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

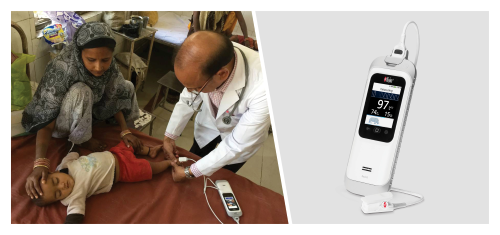
Masimo Rad-G® Helps Clinicians Identify Pediatric Pneumonia in Large Field Trial in India
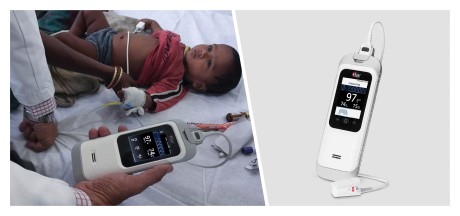
Neuchatel, Switzerland - November 29, 2021 - Masimo (NASDAQ: MASI) today announced the findings of a study published in Clinical Medicine Insights: Pediatrics in which Dr. Harish Kumar and colleagues at IPE Global in New Delhi, India reported on their experience using the Masimo Rad-G® Pulse Oximeter to aid health providers in pneumonia case detection and management in more than 4,500 children under five years who presented with symptoms of acute respiratory infection (ARI). Rad-G is a rugged, portable, handheld Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximeter and noninvasive respiration rate monitor from the pleth (RRp®). The researchers found that Rad-G was "highly acceptable among health workers" and aided the "timely classification and treatment" of pneumonia – helping them achieve correct case management in more than 91% of cases of ARI and reduce the unnecessary use of antibiotics.1
Study author Dr. Kumar commented, "Our decision to choose Rad-G as our pulse oximeter of choice to aid HWCs in pneumonia screening proved to be a good one. The device is easy to use and maintain, even in low-resource settings, and because of its ability to accurately and reliably measure SpO2 and RR, it has the potential to transform the identification and management of pneumonia by healthcare workers, even those who may not be medical doctors. We hope that our study will help convince many more Indian states of the value of integrating use of Rad-G and its technological benefits into their care practices, supporting nationwide efforts to successfully diagnose and treat as many cases of pediatric pneumonia as possible."
As the authors note, pneumonia – one of the most common causes of ARI in children – contributes to 15% of child deaths across the world, with India accounting for 20% of those deaths. In low-resource health settings, where access to diagnostic aids is limited, health workers often rely on manual counts of respiratory rate to inform ARI management decisions. In this trial, researchers evaluated oxygen saturation (SpO2) and respiratory rate (in accordance with WHO guidelines for effective pneumonia management) measured by Rad-G. Given the often "inadequate skills" of front-line healthcare workers in low-resource and rural settings – for example, it was found that the majority of workers at Indian Health and Wellness Centers (HWCs) "lacked knowledge on how to correctly assess a child with cough or difficult breathing" – the authors hoped that Rad-G could help workers more readily diagnose pneumonia, prove to offer good "usability," and ultimately, contribute to India's goal of aggressively reducing child deaths due to pneumonia.
The researchers chose Rad-G from among other available pulse oximeters for a variety of reasons, in particular its integration of respiration rate from the pleth (RRp) and accurate and reliable SpO2. Before proceeding with this larger trial, an initial study at a single tertiary care hospital was conducted in 2019 to evaluate the accuracy of RRp on Rad-G. That study found a 97% association between Rad-G RRp and pediatrician-measured RR, with high sensitivity, specificity, and accuracy.2 The authors also noted Rad-G's long-lasting, rechargeable battery, its LCD screen, and the fact that a single sensor could be used on all children under five years. Following the initial study, Rad-G was introduced at 19 HWCs across seven states in India, and its implementation and utility (including usability and durability) were tracked over 15 months, from June 2019 to August 2020. Over this period, a total of 4,846 children aged 2–60 months with symptoms of ARI visited the facilities. To aid in assessing cases, providers were given abridged training in India's Integrated Management of Neonatal and Childhood Illnesses (IMNCI) program, which classifies children with ARI as having severe pneumonia (SpO2 < 90% or presence of "general danger signs"), pneumonia (fast breathing or chest in-drawing), or neither (none of the above).
Of the 4,846 children, 0.1% were diagnosed with severe pneumonia and 23% with pneumonia. Reviewing cases on a monthly basis, the researchers found that 91.4% of all cases were correctly managed. In addition, 12 children with severe pneumonia, who were referred, would have been missed without the use of Rad-G pulse oximetry.
The researchers concluded, "The pulse oximeter implementation was found to integrate well within a primary healthcare level. The robustness and ease of usability of the device is perhaps the biggest advantage observed, which has led to some of the states budgeting for PO [pulse oximetry] for scale-up in all the districts. A rigorous evaluation in scaled up facilities should be considered by the government. The implementation tentatively demonstrates that a systematic approach to diagnosing pneumonia is likely to improve case management."
The authors also noted, "Considering the importance of hypoxemia and fast breathing as a sign of severe illness, an ideal pulse oximeter is one which functions as a point-of-care device, is durable, affordable, easy to maintain and can deliver rapid, reliable noninvasive SpO2 measurements. A device that measures respiratory rate should also be considered for wider usage given the difficulty among healthcare workers [of] measur[ing] respiratory rate manually. Improving case management of pneumonia at the primary care level by expanding ARI diagnostic aids, while also increasing coverage of IMNCI, strengthening referral pathways, and improving quality of care in referral facilities, will contribute majorly to the SDG [Sustainable Development Goal] goal of reducing under-5 mortality."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Kumar H, Sarin E, Saboth P, Jaiswal A, Chaudhary N, Mohanty J, Bisht N, Tomar S, Gupta A, Panda R, Patel R, Kumar A, Gupta S, Alwadhi V. Experiences from an Implementation Model of ARI Diagnostic Device in Pneumonia Case Management Among Under-5 Children in Peripheral Healthcare Centers in India. Clin Med Insights: Pediatrics. 2021;(15)1-10. DOI: 10.1177/11795565211056649.
- Alwadhi V, Sarin E, Kumar P, Saboth P, Khera A, Gupta S, Kumar H. Measuring accuracy of plethysmography based respiratory rate measurement using pulse oximeter at a tertiary hospital in India. Pneumonia. 2020. 12:4. https://doi.org/10.1186/s41479-020-00067-2.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-G®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-G, contribute to positive clinical outcomes and patient safety; risks that the researchers' conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

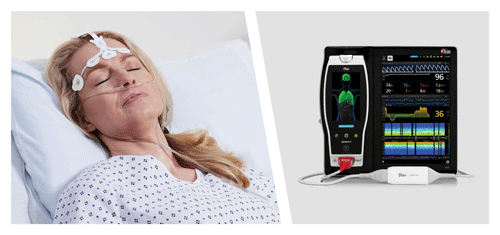
New Study Finds That Use of Masimo SedLine® PSi and DSA May Significantly Reduce Postoperative Delirium
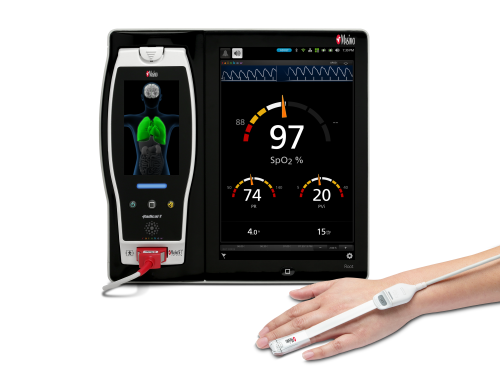
Neuchatel, Switzerland - November 15, 2021 - Masimo (NASDAQ: MASI) today announced the findings of a prospective study published in Frontiers in Neurology in which Dr. Na Xu and colleagues at Capital Medical University in Beijing investigated whether general anesthesia guided by Masimo SedLine® Brain Function Monitoring parameters on Root® could reduce the incidence of postoperative delirium (POD) in patients undergoing carotid endarterectomy (CEA). Using a combination of SedLine's Patient State Index (PSi), an index based on processed electroencephalogram (EEG), and the Density Spectral Array (DSA), which represents the power of the EEG on both sides of the brain, to guide anesthesia during the procedure, the researchers found a significantly reduced risk of postoperative delirium, and concluded that patients "may benefit from the monitoring of multiple EEG parameters during surgery."1
The researchers noted that cerebral blood supply may be "severely disrupted" during CEA, the gold standard treatment for severe carotid stenosis, and that cerebral function is "highly vulnerable" to even brief changes in oxygen and blood supply, as well as to cerebral vascular diseases like carotid stenosis. POD is a "common yet serious" type of geriatric neurological dysfunction associated with worse short- and long-term prognosis and higher healthcare costs. Noting that the incidence of POD is associated with the duration of EEG suppression during surgery, they sought to investigate whether monitoring multiple processed EEG parameters simultaneously to guide anesthesia during a procedure like CEA could positively impact the incidence of POD, compared to use of a single parameter alone.
The authors enrolled 255 patients scheduled for CEA and divided them randomly into an intervention group (n=127, mean age 62) and a standard group (n=128, mean age 63). In the intervention group, general anesthesia was managed using a combination of Masimo SedLine PSi and DSA monitoring (designed to reduce the risk of intraoperative EEG burst suppression); in the standard group, PSi without DSA monitoring was used. In both groups, patients were also monitored with continuous transcranial Doppler ultrasound and near-infrared spectroscopy (NIRS) (designed to avoid perioperative cerebral hypoperfusion or hyperperfusion). The primary outcome was the incidence of POD, measured using the Confusion Assessment Method, during the first three days after surgery. Secondary outcomes were postoperative hospital length of stay (LOS) and other neurologic complications. A team of neurophysiologists independently reviewed the EEG data acquired by SedLine to calculate the cumulative duration of burst suppression for each patient.
The researchers found that the incidence of POD was significantly lower in the intervention group (7.87% of patients) compared to the standard group (28.91% of patients, p < 0.01). Patients in the intervention group also spent significantly less overall time with EEG suppression. There was no significant difference in the incidence of other neurologic complications.
The researchers concluded, "Processed electroencephalogram-guided general anesthesia management, consisting of PSi combined with DSA monitoring, can significantly reduce the risk of postoperative delirium in patients undergoing CEA. Patients, especially those exhibiting hemodynamic fluctuations or receiving surgical procedures that disrupt cerebral perfusion, may benefit from the monitoring of multiple EEG parameters during surgery."
Postoperative delirium is an acute state of mental confusion characterized by alterations in attention, consciousness, and disorganized thinking. A common and serious complication, POD afflicts up to 60% of patients after major surgery,2-5 is most common in the elderly,2-5 and occurs in up to 91% of the critically ill.6 POD is associated both with worse short- and long-term outcomes and higher costs,3,6-9 and numerous medical bodies – including the American Society of Anesthesiologists (ASA), the United Kingdom National Institute for Health and Care Excellence, the American Geriatric Society, and the American College of Surgeons – have made the prevention of POD a public health priority.10-13 The ASA's Brain Health Initiative, dedicated to minimizing the impact of pre-existing cognitive deficits and optimizing the cognitive recovery and perioperative experience for adults 65 years and older undergoing surgery, describes POD as a "major public health issue."14 The incidence of POD has been associated both with preoperative vulnerabilities and – of key importance to studies such as this – the cumulative duration of intraoperative EEG burst suppression. As the current study and others have found, processed EEG monitoring during surgery, by helping clinicians minimize the duration of burst suppression, may lower the rate of POD.1,15-19
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.20 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,21 improve CCHD screening in newborns,22 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.23-26 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,27 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.28 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Xu N, Li L, Wang T, Jiao L, Hua Y, Yao D, Wu J, Ma Y, Tian T, Sun X. Processed Multiparameter Electroencephalogram-Guided General Anesthesia Management Can Reduce Postoperative Delirium Following Carotid Endarterectomy: A Randomized Control Trial. Front Neurol. 12 July 2021. 12:666814. doi: 10.3389/fneur.2021.666814.
- Lipowski ZL. Delirium in the elderly patient. N Engl J Med. (1989) 320:578–82. doi: 10.1056/NEJM198903023200907.
- Khadka J, McAlinden C, Pesudovs K. Cognitive trajectories after postoperative delirium. N Engl J Med. (2012) 367:30–9. doi: 10.1056/NEJMoa1112923.
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. (2014) 383:911–22. doi: 10.1016/S0140-6736(13)60688-1.
- Bin Abd Razak HR, YungWY. Postoperative delirium in patients undergoingtotal joint arthroplasty: a systematic review. J Arthroplasty. (2015) 30:1414–7. doi: 10.1016/j.arth.2015.03.012.
- Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. (2015) 350:h2538. doi: 10.1136/bmj.h2538.
- Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. (1994) 97:278–88. doi: 10.1016/0002-9343(94)90011-6.
- Crocker E, Beggs T, Hassan A, Denault A, Lamarche Y, Bagshaw S, et al. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thoracic Surg. (2016) 102:1391–9. doi: 10.1016/j.athoracsur.2016.04.071.
- Mashour GA, Woodrum DT, Avidan MS. Neurological complications of surgery and anaesthesia. Br J Anaesthesia. (2015) 114:194–203. doi: 10.1093/bja/aeu296.
- American Society of Anesthesiologists. Perioperative Brain Health Initiative Website. (2018). Available online at: https://www.asahq.org/brainhealthinitiative (accessed September 16, 2018).
- Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am College Surgeons. (2016) 222:930– 47. doi: 10.1016/j.jamcollsurg.2015.12.026.
- O’Mahony R, Murthy L, Akunne A, Young J. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Internal Med. (2011) 154:746–51. doi: 10.7326/0003-4819-154-11-201106070-00006.
- National Institute for Health and Care Excellence. Delirium in Adults. London: National Institute for Health and Care Excellence (2014).
- Brain Health. ASA. https://www.asahq.org/in-the-spotlight/brain-health. Accessed 13 Nov 2021.
- Tang CJ, Jin Z, Sands LP, Pleasants D, Tabatabai S, Hong Y, Leung JM. ADAPT-2: A Randomized Clinical Trial to Reduce Intraoperative EEG Suppression in Older Surgical Patients Undergoing Major Noncardiac Surgery. Anesth Analg 2020; 131(4):1228-1236.
- Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesthesia. (2013) 110:98–105. doi: 10.1093/bja/aet055.
- MacKenzie KK, Britt-Spells AM, Sands LP, Leung JM. Processed electroencephalogram monitoring and postoperative delirium: a systematic review and meta-analysis. Anesthesiology. (2018)129:417–27. doi: 10.1097/ALN.0000000000002323.
- Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. (2010) 85:18–26. doi: 10.4065/mcp.2009.0469.
- Whitlock EL, Torres BA, Lin N, Helsten DL, Nadelson MR, Mashour GA, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. (2014) 118:809–17. doi: 10.1213/ANE.000000000000002.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and SedLine®, and the combination of Masimo SedLine processed electroencephalogram parameters ("Combined Parameters") in reducing postoperative delirium. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, SedLine, and the Combined Parameters, contribute to positive clinical outcomes and patient safety; risks that the researchers' conclusions and findings about the Algorithm may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

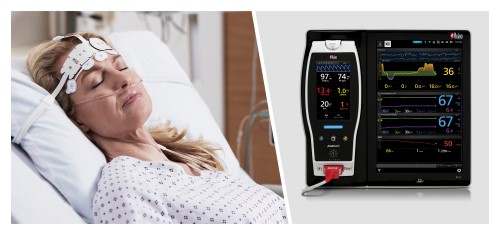
Masimo Root® with a Multimodal Brain Monitoring Algorithm May Improve Postoperative Neurocognition in Elderly Patients
Neuchatel, Switzerland - November 8, 2021 - Masimo today announced the findings of a prospective study published in Frontiers in Aging Neuroscience in which Dr. Shuyi Yang and colleagues at Capital Medical University in Beijing investigated whether Masimo Root® with a multimodal brain monitoring algorithm to manage anesthesia during spinal surgery could improve postoperative cognitive function. In the first study of its kind, the algorithm incorporated measurements from Root, including Masimo SedLine® Brain Function Monitoring, Masimo O3® Regional Oximetry, and ANI® Analgesia Nociception Index. The researchers concluded that managing anesthesia based on the multimodal algorithm “may improve the post-operative cognitive function and brain function connectivity in elderly patients undergoing spinal surgery compared to routine anesthesia management.”1
Noting that perioperative neurocognitive disorder (PND) is common in elderly patients undergoing surgery, and that PND has been associated with levels of sedation, analgesia, and cerebral oxygen saturation, the researchers sought to evaluate whether use of an algorithm designed around related parameters could help improve this population’s postoperative neurocognition. They enrolled 26 patients aged ≥ 65 scheduled to undergo spinal surgery and divided them randomly into an intervention group (n=14) and a control group (n=12). In the intervention group, anesthesia was managed using the algorithm, which incorporated SedLine Patient State Index (PSi) and Spectral Edge Frequency (SEF), O3 regional cerebral oxygen saturation (rSo2), ANI pain index, mean arterial pressure (MAP), end-tidal Co2 (PETCo2), hemoglobin (Hb), and temperature. The control group received routine anesthesia management. To evaluate whether the algorithm improved cognitive function, they a) compared the patients’ Montreal Cognitive Assessment (MoCA) score before and 7 days after surgery, b) analyzed the amplitude of low-frequency fluctuation (ALFF) and brain functional connectivity (FC) after MRI, c) measured serum C-reactive protein (CRP) and lipopolysaccharide levels, and d) analyzed the correlation between FC and changes in inflammatory marker levels.
The researchers found that the mean postoperative MoCA score was higher in the intervention group (24.80 ± 2.09) than in control group (22.56 ± 2.24) (p = 0.04), with no significant difference in the incidence of PND between the groups. (The MoCA score was also higher in the intervention group than in the control group preoperatively, but to a lesser degree than postoperatively.) They also found that patients in the intervention group had significantly increased ALFF values in several brain regions after surgery (p < 0.05) and enhanced FC between the left hippocampus and several regions (p < 0.05), which was negatively corelated with the change in serum CRP (pre- vs. post-intervention) (r = -0.58, p = 0.01).
The authors concluded that “anesthesia management based on multimodal brain monitoring under general anesthesia may improve the postoperative cognitive function and brain function connectivity in elderly patients undergoing spinal surgery compared to routine anesthesia management, as evidenced by increased brain activity (ALFF), enhanced FC, higher MoCA score, and reduced systemic inflammation. The extent of postoperative systemic inflammation was negatively associated with the FC enhancement and may be accompanied by a lower MoCA score. Our findings provide a basis for more effective management of elderly patients who undergo surgery to reduce the risk of cognitive disorders and improve brain function.”
Michael A.E. Ramsay, MD, FRCA, Chair Emeritus of the Department of Anesthesiology and Pain Management at Baylor University Medical Center, commented, “Postoperative neurocognitive disorders (PNDs) are commonly seen in elderly patients, and may be very distressing to the patient and family. This small, prospective, randomized clinical study has demonstrated that precision multimodal monitoring of the brain intraoperatively can result in significantly improved mental status of surgical patients postoperatively. The study patients were maintained at a precise depth of anesthesia, cerebral oxygenation, analgesia, and temperature using the Masimo Root monitor. Postoperatively the MoCA score was statistically higher (p < 0.04) in the study group and the inflammatory marker levels in the brain were significantly reduced (p < 0.05), as well as inflammatory markers systemically (p < 0.01). A MoCA score of 25-30 represents normal cognition and 21-24, 10-20, and 9 and below, mild, moderate, and severe cognitive impairment, respectively.”
Dr. Ramsay continued, “This was a well implemented study, and while it may have been small, it has large implications regarding the value of precision monitoring during surgery and with the potential for application in the intensive care unit (ICU). This may represent a vital advance in the prevention of PND and also the prevention of delirium in ICU patients. Larger studies will be needed to confirm these preliminary data.”
ANI on Masimo Root has not received FDA clearance and is not available for sale in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that Spo2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the Spo2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Yang S, Xiao W, Wu H, Liu Y, Feng S, Lu J, Wang T. Management Based on Multimodal Brain Monitoring May Improve Functional Connectivity and Post-operative Neurocognition in Elderly Patients Undergoing Spinal Surgery. Frontiers Aging Neurosci. 15 July 2021. 13:705287. doi: 10.3389/fnagi.2021.705287.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and Spo2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Root®, SedLine®, O3®, ANI®, and the multimodal algorithm incorporating these measurements (the “Algorithm”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Root, SedLine, O3, and ANI, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings about the Algorithm may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Launches Dual SET® Pulse Oximetry
Neuchatel, Switzerland - November 1, 2021 - Masimo (NASDAQ: MASI) today announced Dual SET® Pulse Oximetry for Root®, a highly versatile patient monitoring and connectivity hub. The first application of Dual SET® Oximetry is a significant advancement to Masimo SET®-guided critical congenital heart disease (CCHD) screening, with the CE marking and European launch of the Masimo SET® MOC-9® module and the addition of the Eve™ CCHD Newborn Screening Application for Root. Together, this combined solution enhances the automation of newborn screenings using Dual SET® Oximetry: two simultaneous measurements of oxygen saturation (SpO2) at pre- and post-ductal sites by the intuitive Eve application, customized to align with a hospital’s CCHD screening protocol.
CCHD affects approximately 2.5 to 3 newborns per 1000 live births1 and requires intervention soon after birth to prevent significant morbidity or mortality; later detection in infants also increases the risk of brain damage.2 Traditionally, newborns were observed for evidence of CCHD by physical assessment and monitoring for common symptoms, but studies have shown that physical assessment of newborns alone can be unreliable and may fail to detect some infants with CCHD before discharge.3-5 Adding screening with pulse oximetry can help clinicians identify CCHD before an infant becomes symptomatic.6 Clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry has been shown in more than 10 CCHD screening studies – representing over 300,000 babies – to increase the effectiveness of screening newborns for CCHD.1,7-16 For example, in a study of almost 40,000 infants, CCHD screening sensitivity increased from 63% with physical exam alone to 83% with physical exam and SET®.1 In another study of more than 120,000 infants – the largest CCHD screening study to date – combined use of clinical assessment and SET® increased screening sensitivity from 77% to 93%.7 Evidence from CCHD studies using SET® has even been used to help establish CCHD screening guidelines used around the world.17
Powered by Masimo SET® pulse oximetry, the Eve CCHD Newborn Screening Application is designed to simplify the CCHD screening process by providing step-by-step visual instructions, animations, and a detailed, easy-to-interpret display of screening results – standardizing and enhancing clinical workflows, improving consistency in screening practices among clinicians, and reducing the possibility of calculation errors. Eve also allows clinicians to incorporate perfusion index into screening, which has been shown to increase sensitivity to the detection of CCHD.18-19
Already available for Radical-7® and Rad-97® Pulse CO-Oximeters®, Eve is particularly well suited for display on Root’s large, high-resolution screen. With its built-in barcode scanner, Root can automatically associate patients with their screening results, and with its integration into the Masimo Hospital Automation™ platform, Root automates the transfer of those results to electronic medical records (EMRs) – eliminating the need for manual charting.
Now, with the addition of the new Masimo SET® MOC-9 module for Root – made possible by another key differentiator of the hub, its advanced, flexible connectivity capabilities – CCHD screening guided by Eve is even more streamlined and efficient: one pulse oximetry sensor can be connected to Root via Radical-7, and a second via the MOC-9 module, allowing for the pre- and post-ductal SpO2 readings needed for screening to be taken simultaneously rather than sequentially, with results conveniently displayed on one screen. This Dual SET® Oximetry technique streamlines the CCHD screening process, improving clinical workflows.
Gerard R. Martin, MD, C.R. Beyda Professor of Cardiology at Children’s National Hospital, said, “As an advocate for congenital heart disease efforts nationally and internationally, I believe Masimo SET® pulse oximetry is an excellent tool for pulse oximetry CCHD screening. Having access to accurate simultaneous pre-ductal and post-ductal measurements helps simplify the process of screening and allows for rapid recognition of discrepancies, ultimately improving newborn care.”
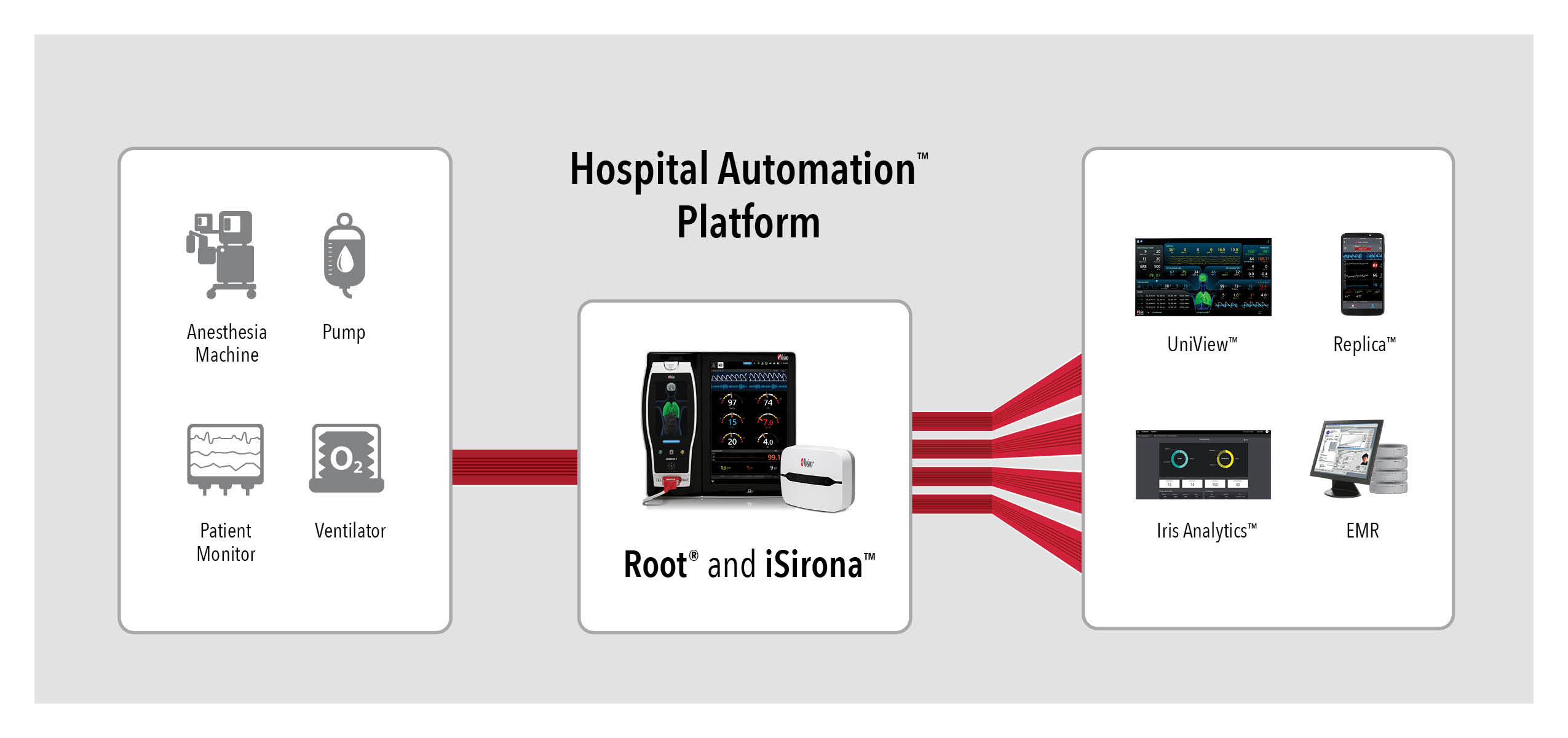
Root is a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide centralized, multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with numerous measurements in addition to dual oximetry Masimo SET®, such as advanced rainbow® Pulse CO-Oximetry measurements, O3® regional oximetry, and SedLine® brain function monitoring, for expanded visibility of patient status. Using Root in combination with the Hospital Automation platform, monitoring data from all connected devices can be automatically charted in EMRs.
Augusto Sola, MD, Vice President of Medical Affairs at Masimo, commented, “As a neonatologist who has worked nationally and internationally in the early diagnosis and treatment of hypoxemic and hyperoxemic conditions that affect neonates in order to improve neonatal survival and quality of life for these fragile infants, I know that Masimo SET® measure-through motion technology’s accuracy and reliability have not only enabled CCHD screening with pulse oximetry, but have helped dramatically reduce retinopathy of prematurity (ROP).200 SET® provides reliable, high-quality monitoring to prevent serious long-term morbidities and is now the standard of care for CCHD newborn screenings and ROP. With the availability of the SET® MOC-9 Module, clinicians can now obtain simultaneous, dual oximetry pre- and post- ductal measurements, using one display, and increase efficiency of CCHD newborn screenings with Root. Furthermore, the Eve application on Root is automated and therefore simplifies and systematizes the screening process. Millions of newborn babies and their families throughout the world will be greatly benefited by this unique solution.”
Eve and the SET® module have not obtained FDA clearance and are not available in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.21 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,20 improve CCHD screening in newborns,1 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.22-24 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,25 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.26 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
- Ewer AK et al. NIHR Health Technology Assessment Programme: Executive Summaries.
- de Wahl Granelli A et al. BMJ. 2009;Jan 8.
- Zhao et al. Lancet. 2014 Aug 30;384(9945):747 54.
- Ewer AK, et al. Lancet Child Adolesc Health. 2017;1(2):88 90.
- Zhao et al. Lancet. 2014;384(9945):747-54.
- Gunaratne CR et al. Sri Lanka J Child Health. 2021;50(1):04-11.
- Slitine N et al. Int J Neonatal Screen. 2020;6(53).
- Ewer AK et al. Lancet. 2011;378(9793):785-94.
- de-Wahl Granelli A et al. Acta Paediatr. 2007;96(10):1455-9.
- Meberg A et al. J Pediatr. 2008;152:761-5.
- Schena F et al. J Pediatr. 2017;183:74-79.
- Hamilçıkan S, Can E. J Perinat Med. 2018;46(2):203-207.
- Jawin V et al. PLoS One. 2015;10(9):e0137580.
- Gopalakrishnan S et al. Med J Armed Forces India. 2021;77(2):214-219.
- Kemper et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011 Nov;128(5):e1259-67. doi: 10.1542/peds.2011-1317.
- Siefkes H, et al. Am J Perinatol. 2020; 37(2):158 165.
- Uygur O et al. Pediatr Neonatol. 2019;60(1):68 73.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®, Root®, Eve™, SET® MOC-9® Module, and the dual oximetry screening tool they combine to provide (the “Solution”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, Root, Eve, SET® MOC-9 Module, and Solution, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Recommends the Use of Masimo PVi® to Guide Fluid Administration During Major Oncosurgery with Lung Ultrasonography
Irvine, California - October 18, 2021 - Masimo (NASDAQ: MASI) today announced the findings of an abstract presented at ANESTHESIOLOGY 2021 in San Diego, in which Drs. Anita Kulkarni and Arti Dalal at the Rajiv Gandhi Cancer Institute in New Delhi, India evaluated the use of noninvasive, continuous Masimo PVi® as part of goal-directed fluid therapy (GDFT) to guide intraoperative fluid administration during major oncosurgery in which lung ultrasonography (LUS) is used to diagnose extravascular lung water (EVLW). The researchers found that use of PVi, as a dynamic, continuous method of managing fluid administration, led to patients receiving less fluid and having fewer B-lines (a measure of EVLW), with no significant decrease in postoperative perfusion or oxygenation, compared to use of a central venous pressure (CVP)-guided fluid administration protocol.1
Noting that conventional invasive methods of guiding fluid administration (such as CVP) during major oncosurgery may increase EVLW, which can lead to postoperative cardiorespiratory complications, the authors investigated whether use of a noninvasive, dynamic, continuous method—GDFT guided by Masimo PVi—could improve fluid management by reducing the amount of fluids administered and B-lines as measured by LUS. To evaluate PVi, they compared two groups of adult patients undergoing major oncosurgery with LUS: a group of 60 patients whose fluid administration was guided by PVi as part of GDFT and a group of 59 patients whose fluid administration was guided by CVP. Their primary outcome was detection of EVLW indicated by the total number of B-lines, and their secondary outcome was adequacy of perfusion by lactate levels at the end of surgery. The authors also measured arterial blood gas samples at baseline and the end of surgery to evaluate oxygenation.
Comparing the amount of fluids administered, the researchers found there were significantly fewer total crystalloids given in the PVi group (1875.8 ± 593.9 ml) compared to the CVP group (2132.6 ± 504.5 ml; p = 0.012), as well as significantly less colloid provided in the bolus (PVi group: 584 ± 358.5 ml; CVP group: 778.2 ± 242.2 ml; p = 0.001). They found that 23.3% of PVi-group patients had “mild” B-lines and 5.0% “moderate” B-lines, compared to 44.1% mild and 5.1% moderate in CVP-group patients (p = 0.042). At the end of surgery, they found that in the CVP group, there was a statistically significant decrease (p < 0.05) in partial pressure of oxygen in arterial blood (PaO2) and the ratio of arterial blood oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2), compared to baseline levels; in the PVi group, the parameters were comparable to baseline after surgery, with no significant difference.
The authors concluded, “We recommend lung ultrasonography after completion of major oncosurgeries to detect EVLW and intraoperative PVi-guided GDFT as these patients received less fluids, had less B-lines (mild) and no decrease in PaO2 or PaO2/FiO2 levels at [the] end of surgery.”
In the U.S., PVi is cleared as a noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Kulkarni A, Dalal A. Lung Ultrasonography for Diagnosing Extravascular Lung Water in Major Oncosurgeries. Presented at ANESTHESIOLOGY 2021, San Diego, California, October 9, 2021. Abstract #A1067.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of PVi® and the new study recommending the use of PVi (the “Study”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including PVi, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusion and recommendation based on the Study may not be accurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo and University Hospitals Partner to Combat Nurse Burnout
Masimo Patient SafetyNet™ Remote Monitoring and Supplemental Alarm System Improves Nursing Workflow and Reduces Nursing Workloads
Irvine, California - October 4, 2021 - Masimo (NASDAQ: MASI) today announced that University Hospitals (UH) of Cleveland is using Masimo Patient SafetyNet™, a remote monitoring and supplemental alarm system, to combat nurse burnout by improving workflows and reducing workloads. As the COVID-19 pandemic continues to strain healthcare infrastructure around the world, hospitals are dealing with higher than usual rates of nurse turnover. Patient SafetyNet offers a technological approach to supporting nursing workflows, as both UH’s experience and clinical evidence have shown.
During the pandemic, more than 80% of hospitals have reported an increase in nurse turnover.1 In a survey of more than 20,000 nurses conducted in 2020, 18% intended to resign, 50% responded that work was negatively impacting their health, and 47% reported that insufficient staffing contributed to their desire to leave the profession.2 Nurse staffing shortages have been associated with both increased costs and, crucially, decreased patient safety: A survey of 138 facilities covering more than 120,000 nurses found that the average cost of turnover ranged from $37,400 to $58,400 per nurse.3 And an observational, retrospective study of almost 200,000 patients across 43 hospital units found a significant association between increased patient mortality and nurse staffing below target levels.4
In a newly released testimonial, available here, UH – one of the largest health systems in Ohio – shows how their experience with continuous remote monitoring using Patient SafetyNet is positively impacting their nursing practices and the quality of care nurses are able to provide while improving efficiency and staff satisfaction. Within three weeks of implementing Patient SafetyNet, for example, UH found that the average time between obtaining a patient's vital signs at the point of care and documenting them in the electronic medical record (EMR) had decreased from more than 60 minutes to less than 5 minutes, resulting in a time savings of one FTE per shift.
UH first implemented Patient SafetyNet in 2016 following an incident in which an unnoticed, rapid deterioration in a patient's condition resulted in emergency resuscitation and ICU transfer. Jenna Zarack, a nurse at UH's Geauga Medical Center, said that Patient SafetyNet is "a great way to protect patients; it's a great way to protect nurses, and for nurses to be able to help their patients in a fast, immediate response, so we don’t get to a code, we don’t get to a rapid response situation." Sara Knowles, MSN, Clinical Nurse Specialist at UH, added, "The technology allows us to have eyes on our patients 24/7, which has proven especially valuable during our nursing shortage and the global pandemic."
When asked why UH is now planning to expand use of Patient SafetyNet – which is already in use in 8 med-surg units across 5 UH hospitals – Michelle Hereford, UH’s Chief Nursing Executive, explained, "We've found that Patient SafetyNet's continuous surveillance monitoring and automation capabilities help lower the burden on nurses and support them in providing quality care for every patient and catching potential patient deterioration before it's too late. Coupled with the improvements we've seen in efficiency, staff confidence, and staff satisfaction since installing the system, expanding use of Patient SafetyNet is frankly a no-brainer."
In addition to the experiences of Patient SafetyNet users like UH, there is significant clinical evidence for the efficiency and workflow benefits the centralized surveillance monitoring system offers, such as:
- In a recent study published in the Journal of PeriAnesthesia Nursing researchers evaluated the utility and impact of Patient SafetyNet by surveying nurses before and after implementation and found that use of the system decreased the number of physical assessments, resulting in a reduction in nursing workload, and also recommended the use of continuous respiratory rate and oxygen saturation monitoring (which was implemented as part of the system) after general anesthesia, for patients’ safety.5
- In a study conducted at Dartmouth-Hitchcock Medical Center, researchers investigated the impact of Patient SafetyNet on clinical workflow, and found a significant decrease in the time required to obtain and record vital signs (with mean assessment time dropping from 179 seconds to 129 seconds, saving an average of 3 hours a day in a 36-bed unit). They also found a significantly higher rate of patient data being accurately filled out in electronic medical records (EMRs), and an overall "very high" staff satisfaction rate with the system. The researchers concluded, "The enhanced monitoring system received high staff satisfaction ratings and significantly improved key clinical elements related to early recognition of changes in patient state, including reducing average vital signs data collection time by 28%, increasing patient monitoring time (rate ratio 1.22), and availability and accuracy of patient information."6
Patient SafetyNet, powered by the Masimo Hospital Automation™ platform, displays real-time information from connected Masimo and third-party devices at central stations and on remote smart devices using Replica™, helping clinicians keep track of up to 200 patients per view station at a time. In addition, the system allows actionable alarms and alerts to be sent directly to clinicians, regardless of their location. Automatic escalation is provided for unattended alarms, helping to facilitate better awareness when patients need assistance. In addition to the workflow and workload reductions it offers, use of Patient SafetyNet for continuous remote supplemental monitoring with Masimo SET® pulse oximetry has been shown to have a significant impact on patient outcomes. For example, in multiple studies conducted over ten years at Dartmouth-Hitchcock, researchers found dramatic reductions in rapid response rescue events, ICU transfers, and zero deaths or preventable brain damage due to opioids in monitored patients.7-10
In addition to its powerful connectivity and automation features, which drive improvements for patients and nurses alike, Patient SafetyNet also offers powerful, integrated data aggregation and reporting software, Iris® Analytics. Designed to transform patient data into actionable information on hospital performance, floor protocol, and individual patient progress, Iris Analytics allows users to analyze past alarm events, notifications, and much more, to gain insight into their institution's performance – and model improvements to support the reduction of nuisance alarms and thus improve workflows. Based on analysis of even just one to two weeks of past alarm event data, the Limit Analysis Report helps clinicians simulate alarm scenarios using theoretical alarm thresholds and delays, providing key data to help them evaluate and refine their alarm strategies to better meet their needs.
UH Chief Clinical Transformation Officer, Peter Pronovost, MD, commented, "Masimo Patient SafetyNet has been a valuable component in advancing our Zero Harm initiative within the University Hospitals system. It's allowed us to increase the efficiency of our caregivers, reduce clinical errors, and help us focus on the timely delivery of compassionate care to our patients. It has also been a critical monitoring partner during the pandemic, when the condition of our COVID patients can dramatically change."
Joe Kiani, Founder and CEO of Masimo, said, "As the pandemic continues, hospitals and other healthcare facilities are coming up against resource limitations across the board – from equipment, supplies, and beds to doctors and administrative staff – but the shortage of qualified nurses, due to burnout, may be the most significant challenge of all. Masimo has spent more than 25 years improving monitoring and automation technologies designed not only to improve patient outcomes and reduce the cost of care, but to make it easier for nurses to do their jobs effectively and efficiently. We hope that more and more institutions, like University Hospitals, will have the opportunity to experience the benefits that Patient SafetyNet can provide for patients and staff alike."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.11 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,12 improve CCHD screening in newborns,13 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,14 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.15 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- https://www.incrediblehealth.com/blog/study-covid-19-anniversary-nurse-impact/
- https://www.nursingworld.org/practice-policy/work-environment/health-safety/disaster-preparedness/coronavirus/what-you-need-to-know/year-one-covid-19-impact-assessment-survey/
- https://rnbsnonline.unm.edu/articles/high-cost-of-nurse-turnover.aspx
- Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011 Mar 17;364(11):1037-45. doi: 10.1056/NEJMsa1001025. PMID: 21410372.
- Ishikawa M, Sakamoto A. Patient SafetyNet for the Evaluation of Postoperative Respiratory Status by Nurses: A Presurvey and Postsurvey Study. J PeriAnesth Nursing. DOI: https://doi.org/10.1016/j.jopan.2020.03.005.
- McGrath S, Perreard I, Garland M, Converse K, and Mackenzie T. Improving patient safety and clinician workflow in the general care setting with enhanced surveillance monitoring. J Biomed Health Infor. DOI 10.1109/JBHI.2018.2834863.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks that Masimo Patient SafetyNet may fail to perform satisfactorily despite positive clinical evidence and its past success at UH; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Evaluates the Feasibility of Using Masimo EMMA® Capnography on Mechanically Ventilated Neonates
Tokyo, Japan – September 27, 2021 - Masimo (NASDAQ: MASI) today announced the findings of a study published in the European Journal of Pediatrics in which Dr. Masashi Hotta and colleagues at the Osaka Women's and Children's Hospital in Japan found that the Masimo EMMA® Portable Capnograph “may be considered an effective monitoring device” for mechanically ventilated preterm infants (neonates).1
Noting the importance of maintaining an appropriate range of partial pressure of arterial carbon dioxide (PaCO2) in preterm infants, especially while undergoing mechanical ventilation in the neonatal intensive care unit (NICU), the researchers sought to evaluate whether noninvasively monitoring end-tidal carbon dioxide (EtCO2) with EMMA could help clinicians maintain neonatal PaCO2 in the delivery room. They chose EMMA not only because of its portability but because it offers a solution with a small dead space (1 mL). The researchers enrolled 40 neonates (gestational age of 26+0 to 31+6 weeks) who required intubation in the delivery room (the EMMA monitoring group) and compared their PaCO2 value, either at admission to the NICU or 2 hours after birth, with that of 43 infants who did not undergo EMMA monitoring (the historical control group). They defined “appropriate” PaCO2 as 35 – 60 mmHg, as measured using a blood gas analyzer.
The researchers found that the proportion of infants with appropriate PaCO2 was greater in the EMMA group than in the control group (80% vs. 42%, p = 0.001). Stratified according to birth weight (< 1000 g vs. > 1000 g), they found that in smaller neonates, there was no significant difference in the proportion of infants with appropriate PaCO2 between groups, but in the larger cohort, the rate of appropriate PaCO2 was significantly higher in the EMMA group: 93% vs. 44%, p < 0.001.
The study authors concluded that EMMA "facilitated the maintenance of an appropriate PaCO2 for mechanically ventilated pre-term infants, especially infants with birth weight ≥ 1000 g, in the delivery room." They noted that the main strength of their study was that they "collected intervention data prospectively and showed the feasibility of using a portable capnometer during resuscitation of intubated preterm infants" – the first study of its kind.
EMMA provides seamless mainstream capnography for patients of all ages in a compact, easily portable device. The instrument requires no routine calibration and minimal warm-up time, with accurate EtCO2 and respiration rate measurements and continuous EtCO2 waveforms displayed within 15 seconds.
EMMA does not have FDA clearance for neonates.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Hotta M, Hirata K, Nozaki M, Mochizuki N, Hirano S, and Wada K. Feasibility of portable capnometer for mechanically ventilated preterm infants in the delivery room. Eur J Ped. 2021. DOI:10.1007/s00431-021-04246-1.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo EMMA®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo EMMA, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo CEO Joe Kiani Appointed to President’s Council of Advisors on Science and Technology
Irvine, California – September 22, 2021 - Masimo (NASDAQ: MASI) announced today that Founder, Chairman, and CEO Joe Kiani has been appointed by President Joe Biden to the President's Council of Advisors on Science and Technology (PCAST). The President's video announcement can be found here.
Since 1933, with the creation by President Franklin D. Roosevelt of a Science Advisory Board, each President has established an advisory committee of scientists, engineers, and health professionals. Created by Executive Order, President Biden's PCAST will advise him on matters involving science, technology, education, and innovation policy. The Council also provides the President with scientific and technical information needed to inform public policy relating to the American economy, the American worker, national and homeland security, and more. In particular, President Biden has asked PCAST to consider such pressing topics as how the pandemic can inform public health needs and how scientific and technological breakthroughs can help address climate change. The 30 members of the Council, the most diverse in history, include distinguished individuals from sectors outside the Federal Government with diverse perspectives and expertise in science, technology, education, and innovation, including 20 elected members of the National Academies of Sciences, Engineering and Medicine, five MacArthur "Genius" Fellows, two former Cabinet secretaries, and two Nobel laureates. More details about PCAST can be found here.
Joe Kiani commented, "Thank you, President Biden, for this appointment. I am excited to work with this incredible PCAST group and others to explore ways that science and technology, and policies that can shape them, can improve healthcare, the environment, innovation, and equity in our country and the world."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Launches Single-patient-use rainbow® SuperSensor™
One Patient, One Sensor, 12 Parameters: Breakthrough Technology Offers Advanced Physiological Insight in One Comprehensive, Convenient, Noninvasive Fingertip Solution
Neuchatel, Switzerland – September 13, 2021 - Masimo (NASDAQ: MASI) announced today the CE marking and commercial launch in Europe of the single-patient-use adhesive rainbow® SuperSensor™, compatible for use with both Masimo and third-party monitors with Masimo rainbow® technology inside. In an industry first, the comprehensive, convenient, and multi-purpose SuperSensor uses 12 LEDs to simultaneously offer 12 blood constituent parameters noninvasively and continuously: SET® oxygen saturation (SpO2), total hemoglobin, SpHb®, carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), oxygen reserve index (ORi™), PVi®, RPVi™, pulse rate (PR), respiration rate (RRp®), perfusion index (Pi), fractional oxygen saturation (SpfO2™), and oxygen content (SpOC™)—all on the same single-patient-use adhesive sensor. By allowing clinicians to noninvasively and continuously monitor so many different physiologic indicators simultaneously, the SuperSensor offers the ability to assess the patient’s status continuously.
At the core of the SuperSensor is Masimo SET® pulse oximetry, which has been clinically proven to help care teams enhance patient safety and improve patient outcomes; in fact, more than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies in clinical settings and motion and low perfusion conditions, providing clinicians with increased sensitivity and specificity to make critical care decisions.1 SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce deaths due to opioid overdose, while also reducing rapid response team activations, ICU transfers, and the cost of care.4-7
Continuous hemoglobin monitoring with SpHb as part of patient blood management programs has been found to help clinicians improve outcomes in both high- and low- blood loss surgeries, such as reducing the percentage of patients receiving transfusions,8 reducing the units of red blood cells transfused per patient,9-10 reducing the time to transfusion,11 and reducing costs.12 The utility of PVi, a measure of the dynamic changes in perfusion index that occur during the respiratory cycle, as an indicator of fluid responsiveness, has been demonstrated in more than 100 independent studies.13 Use of SpHb and PVi together, as part of an integrated goal-directed therapy protocol for fluid management and blood administration, has even been shown to help clinicians reduce mortality 30 and 90 days after surgery, by 33% and 29%, respectively.14
ORi is a noninvasive and continuous trending index that extends oxygen monitoring of patients receiving supplemental oxygen. By monitoring oxygenation beyond the upper limits of conventional pulse oximetry, ORi offers the potential for advanced warning of hypoxemia, during preoxygenation and intubation procedures, and of hyperoxia, in patients receiving greater concentrations of supplemental oxygen than clinically required. For example, in a study of pediatric patients undergoing general anesthesia with orotracheal intubation, researchers found that ORi detected impending desaturation in a median of 31.5 seconds before noticeable changes in SpO2 occurred.15 A study evaluating the ability of ORi to predict mild hypoxemia during endotracheal intubation found that the time between decrease in ORi and subsequent decrease in SpO2 “may allow preventive action,” and that a higher ORi value during preoxygenation was “independently protective against hypoxemia.”16 In another study, researchers found that monitoring adult ICU patients with ORi significantly reduced the time these critically ill patients spent with moderate hyperoxia, compared to monitoring with oxygen saturation (SpO2) alone.17
SpMet helps clinicians noninvasively and continuously monitor methemoglobin levels in the blood.18 Elevated methemoglobin levels can be caused by many drugs given in hospitals, including inhaled nitric oxide (iNO) therapy,19-20 which has been used as a potential treatment for lung complications associated with COVID-19. SpMet may be an important monitoring tool during iNO therapy.
Dr. Max Jonas, Consultant in Intensive Care Medicine and Anesthesia at University Hospitals, Southampton, UK, commented, “Critically ill patients are frequently hemodynamically unstable, with variable oxygen delivery, which may be inadequate and lead to a cumulative oxygen debt, especially with noradrenaline infusions. Clinically this makes continuous monitoring and optimization of oxygen delivery using hemoglobin, fluid responsiveness, and oxygen saturation extremely important. It is also clinically invaluable being able to recognize impaired oxygen carriage, and hence content in the blood, for instance the methemoglobinemia generated by inhaled nitric oxide therapy, which we are currently frequently using during the treatment of COVID-19 pneumonitis and also for pulmonary hypertension.”
SpCO enables quick and noninvasive monitoring of carbon monoxide levels in the blood, and may lead to the identification of elevated CO levels that might otherwise go unnoticed in front-line settings such as fire rescue and mass casualty scenarios.21,22 Studies of emergency room patients have shown that SpCO may be a valuable tool for monitoring a large number of patients for possible CO exposure.23,24 For example, in a study of emergency room patients, of 32 patients diagnosed with CO poisoning, 22 would not have been identified without SpCO monitoring.25
By making SpMet, SpCO, and SpfO2 available on the same sensor, the SuperSensor provides a more complete picture of oxygenation in the presence of potential dyshemoglobin interference. Fractional oxygen saturation (FO2Hb) provides a measure of the fraction of total hemoglobin that is currently oxygenated, as opposed to SpO2, functional oxygen saturation, which measures the fraction of hemoglobin that is oxygenated based on an estimation of the effective hemoglobin available (hemoglobin capable of being oxygenated). In healthy individuals, FO2Hb is often similar to SpO2, but when dyshemoglobin levels are elevated, FO2Hb may be more representative of the total oxygen-carrying capacity of hemoglobin than SpO2. In the presence of dyshemoglobins, SpO2 may appear “normal,” but SpfO2—a noninvasive, continuous measurement of FO2Hb—may provide more insight into a possible oxygenation impairment. Combined with the ability to monitor SpCO and SpMet on the same sensor, clinicians now have additional information to help determine if a dyshemoglobin species is responsible, and intervene appropriately.
Dr. Anne Booth, Consultant in Neuroanaethesia and PHEM at Cambridge University Hospitals, and Joint Clinical Lead – Adult Critical Care Transfers, East of England, stated, “When assessing patients’ oxygenation, we need to be prepared for the unknown, especially in Emergency and Critical Care. If there are patients that have elevated carboxyhemoglobin from prior history of smoking or from carbon monoxide exposure, their oxygen content would be impaired. Similarly, with inhaled nitric oxide therapy, which is commonly used for COVID-19, patients may be subject to high methemoglobin levels. Parameters provided on the Masimo SuperSensor, like oxygen content (SpOC) and fractional oxygen saturation (SpfO2), can help us identify the source of diminished oxygen delivery so we can react accordingly.”
Dr. Aryeh Shander, Anesthesiologist and an expert in patient blood management, commented, “A key focus in our care for critically ill patients during surgery and beyond is to minimize oxygen consumption and maximize oxygen utilization. The information now available noninvasively, via a pulse oximetry-like sensor, on total hemoglobin concentration, fluid responsiveness, dyshemoglobin presence, fractional oxygen saturation, oxygen content, and more, can give us much needed and critical information to help in providing the best clinical judgements. The ultimate goal is to improve the patient’s outcome and not just treat a number.”
Dr. Kiyoyuki Miyasaka, Anesthesiologist at the National Center for Child Health and Development, Tokyo, Japan, noted, “Pulse oximetry has progressed greatly since its invention in Japan in the 1970s. With the latest sensors from Masimo, clinicians now have greater visibility of the patient’s overall oxygen delivery, helping us better understand the underlying physiology impacting their condition. I look forward to seeing how Masimo pulse oximetry can further improve patient care.”
Joe Kiani, Founder and CEO of Masimo, said, “The SuperSensor represents a key milestone in Masimo’s continued innovation journey, giving clinicians access to 12 breakthrough noninvasive measurements in a single, convenient, and comprehensive sensor as part of our RD rainbow SET® sensor family—while easing concerns about cross-contamination because of being a single-patient-use, adhesive product. I am proud of our team for delivering this innovation to the medical community.”
****
SpHb and SpMet monitoring are not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision-making. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgement considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples. SpCO monitoring is not intended to be used as the sole basis for making diagnosis or treatment decisions related to suspected carbon monoxide poisoning. It is intended to be used in conjunction with additional methods of assessing clinical signs and symptoms.
The accuracy of PVi in predicting fluid responsiveness is variable and influenced by numerous patient, procedure, and device-related factors. PVi measures the variation in the plethysmography amplitude but does not provide measurements of stroke volume or cardiac output. Fluid management decisions should be based on a complete assessment of the patient’s condition and should not be based solely on PVi. In the U.S., PVi is cleared as a noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients.
ORi, RPVi and SpfO2 have not received FDA 510(k) clearance and are not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,26 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.27 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Published clinical studies on PVi, with varying results and outcomes, can be found on our website at http://www.masimo.com/evidence/pulse-oximetry/pvi. Studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Szmuk P et al. Anesthesiology. 2016; 124:00-00.
- Hille H, Le Thuaut A, Canet E, Lemarie J, Crosby L, Ottavy G, Garret C, Martin M, Seguin A, Lamouche-Wilquin P Morin J, Zambon O, Miaihle AF, Reignier J, Lascarrou JB. Oxygen reserve index for noninvasive early hypoxemia detection during endotracheal intubation in intensive care: the prospective observational NESOI study. Ann. Intensive Care. 2021 11:112. DOI: 10.1186/s13613-021-00903-8.
- Lasocki S, Brochant A, Leger M, Gaillard T, Lemarié P, Gergaud S, and Dupré P. ORi monitoring allows a reduction of time with hyperoxia in critically ill patients: the randomized control ORi study. Intensive Care Med. 13 Aug 2019. https://doi.org/10.1007/s00134-019-05732-9.
- Annabi E et al. Severe Methemoglobinemia Detected by Pulse Oximetry. Anesth Analg. 2009 Mar;108(3):898-9.
- Riou Y et al. Pediatric Research. 1998. 43, 295-295.
- U.S. Food & Drug, Consumer Updates, Benzocaine and Babies: Not a Good Mix.
- Augustine JJ. JEMS. 2007 May;64-71.
- Bledsoe BE et al. Prehosp Emerg Care. 2010 Jan-Mar;14(1):131-3.
- Suner S et al. J Emerg Med. 2008 May;34(4):441-50.
- Roth D et al. Ann Emerg Med. 2011 Jul;58(1):74-9.
- Roth D et al. Int J Clin Pract. 2014 Oct;68(10)1239.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo rainbow® SuperSensor™ and the utility of its 12 provided parameters. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo rainbow® SuperSensor, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Investigates the Ability of Masimo ORi™ to Provide Early Warning of Hypoxemia During Endotracheal Intubation in ICU Patients
Researchers Found That Use of ORi May Facilitate "Preventive Action" Against Hypoxemia
Neuchatel, Switzerland – September 6, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a prospective, blinded observational study published in Annals of Intensive Care in which Dr. Jean-Baptiste Lascarrou and colleagues at the Centre Hospitalier Universitaire in Nantes, France evaluated the ability of Masimo ORi™ to predict mild hypoxemia during endotracheal intubation (ETI) in ICU patients.1 They concluded that the time between decrease in ORi and subsequent decrease in oxygen saturation (SpO2) "may allow preventive action," and that a higher ORi value during preoxygenation was "independently protective against hypoxemia."
ORi, available outside the U.S., is a noninvasive and continuous trending index that extends oxygen monitoring on patients on supplemental oxygen. Enabled by the multi-wavelength rainbow® Pulse CO-Oximetry platform, ORi is provided alongside oxygen saturation (SpO2) measured by clinically proven Masimo SET® pulse oximetry.
Noting the importance of optimizing preoxygenation in patients needing ETI, the researchers sought to evaluate whether ORi could provide early warning of impending hypoxemia during the procedure, because ORi "supplies information beyond the range explored by SpO2." Of the 51 patients who met the inclusion criteria, ORi (alongside SpO2) was monitored using Masimo Radical-7® Pulse CO-Oximeters® and rainbow® fingertip sensors when preoxygenation began. Values were recorded every two seconds, and attending clinicians were not aware of these values. The primary endpoint measured was the time between ORi decreasing below 0.4 and the onset of mild hypoxemia (defined as SpO2 < 97%). Secondarily, the investigators evaluated whether a decline in ORi during preoxygenation predicted the occurrence of mild hypoxemia during ETI.
Analyzing areas under the ROC curve, the researchers found that ORi during preoxygenation predicted SpO2 < 97% during intubation (0.73; 95% confidence interval of 0.58 – 0.88). By contrast, SpO2 at the end of preoxygenation was "poorly predictive" (0.54; 95% CI 0.40 – 0.67). Using univariate analysis, they found that a higher ORi value during preoxygenation was associated with a less frequent occurrence of SpO2 < 97% (odds ratio of 0.09; 95% CI 0.01 – 0.69, p = 0.0199) and that "the highest ORi value during preoxygenation remained significantly associated with a lower risk of SpO2 < 97% during ETI, after adjustment for ETI duration and BMI (odds ratio of 0.76; 95% CI 0.61 – 0.95, p = 0.0141)."
The researchers concluded, "The median time between the ORi decrease below 0.4 and the SpO2 decrease below 97% during the apneic period was 81 seconds [34–146]. A higher ORi during preoxygenation was independently associated with a lower risk of mild hypoxemia (SpO2 < 97%)."
The researchers noted that the median 81 seconds of forewarning "may allow immediate intubation, early face-mask ventilation, insertion of a supraglottic device, or a call for help in the event of intubation difficulties." In addition, they noted that “ORi monitoring can help identify patients who do not increase their oxygen reserve despite preoxygenation and are therefore at [increased] risk of desaturation during ETI. These patients may benefit from a longer preoxygenation period and/or a change in device." The authors further noted that “an ORi decline might lead to the detection of a fault in the preoxygenation technique such as an insufficient oxygen flow rate or major leaks."
As a study limitation, the authors noted that their findings cannot be generalized to ICU patients who did not meet the inclusion criteria, as those patient cohorts remain to be evaluated. The inclusion criteria for this study were ICU admission with a need for ETI and an SpO2/FiO2 ratio above 214. The SpO2/FiO2 ratio was measured during noninvasive ventilation or high-flow oxygen therapy; for conventional oxygen therapy, the fraction of inspired oxygen (FiO2) was calculated as FiO2 = 0.21 + O2 flow [rate in L/min] x 0.03.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Hille H, Le Thuaut A, Canet E, Lemarie J, Crosby L, Ottavy G, Garret C, Martin M, Seguin A, Lamouche-Wilquin P Morin J, Zambon O, Miaihle AF, Reignier J, Lascarrou JB. Oxygen reserve index for noninvasive early hypoxemia detection during endotracheal intubation in intensive care: the prospective observational NESOI study. Ann. Intensive Care. 2021 11:112. DOI: 10.1186/s13613-021-00903-8.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks that findings in the new study on Masimo ORi's ability to predict hypoxemia during ETI cannot be generalized; risks that researchers’ conclusion on the practical applications of Masimo ORi’s forewarning ability may not be accurate; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Partners with Penington Institute to Raise Awareness of Harm from Prescription Opioid Overdose
To mark International Overdose Awareness Day (IOAD) on August 30, 2021, Masimo (NASDAQ: MASI), a global medical technology company, announced today its commitment to raising awareness of the risks of overdose from prescription opioids, even when used as directed.

Yvonne Gardner standing beside her son's widow, Madilyn Stewart. Madilyn was married to Parker for <4 months when he died in his sleep of an opioid overdose, despite taking only half the amount prescribed by his doctor.
In December 2016, following a routine tonsillectomy, healthy, 21-year-old Parker Stewart took just half of his prescribed dose of opioids and suffered from opioid overdose in his sleep. Tragically, he never woke up. This could have been prevented. His mother, Yvonne Gardner, pleads, "I wish I had known it was a problem – I didn't know it could happen. I Googled a lot of stuff before the surgery – but I never Googled 'tonsillectomy fatalities,' because you just don’t think that such a routine surgery would end up this way."
Parker's story is just one example of a preventable death from opioid overdose that happens tens of thousands of times each year around the world.1 Awareness and education are important to helping prevent others. In honor of IOAD, Masimo is launching an educational website, www.OpioidSafety.org, which shares information about the potential side effects of prescription opioid painkillers, who is at risk, and how to protect yourself. Furthermore, Masimo, in partnership with the Penington Institute – the founder of IOAD – has committed to funding comprehensive research and reporting on the prevalence and impact of prescription and non-prescription opioid overdoses. The reports will be made publicly available at no charge, with the first report, focused on the UK, expected to be available in 2022.
In the latest figures for 2020, published by the UK Office for National Statistics (ONS), drug-related deaths recorded in England and Wales reached the highest levels since records began in 1993. Of the 2,263 deaths recorded, approximately half involved opioids.2 Already known to be highly addictive, opioids also have serious side effects, including slowed or stopped breathing – which can lead to cardiac arrest, brain damage, or death.3 Harm can occur even when opioids are taken as prescribed.4
Opioid painkillers are prescribed for pain relief and are commonly used by patients during and after surgery, as well as by those experiencing chronic pain. Opioid prescriptions have increased steadily in the UK, with over 50 million opioid prescriptions written in the UK last year alone.2 Importantly, about 30% of those taking a prescription opioid painkiller don’t realize they’re taking an opioid.5 Last year, more people died from opioids than car accidents.6
Dr. Mike Durkin, a Senior NHS Advisor on Patient Safety Policy and Leadership for the National Institute for Health Research (NIHR) Imperial College Patient Safety Translational Research Centre, said, "Patients recovering from surgery still need pain relief using opioid drugs after they are discharged from hospital and return home. However, these drugs have significant side effects, particularly on the depression of breathing, which without urgent intervention can result in serious harm or death. The technology is now available to monitor the impact of opioids on breathing and it is vital that patients are given the opportunity to easily and continuously monitor their oxygen levels and vital signs while taking these medications at home. This will greatly improve the safety of patients while rehabilitating at home."
John Ryan, CEO of the Penington Institute, a leader in public health and safety, and immediate past President of London-based Harm Reduction International, said, "Opioid overdose is a complex problem driven by multiple reasons, and as a result, the true scale is not fully reported or understood. However, just because a problem is underreported, it doesn’t mean it doesn't exist. With incidences of opioid deaths continuing to increase year-on-year, there is an urgent need for better research into treatment and care involving opioids to identify current gaps in care and to help protect people from avoidable harm."
Joe Kiani, Founder and CEO of Masimo, said, "Too many lives are being lost. Many people immediately assume that an overdose is connected only to the use of illicit drugs or taking more opioids than prescribed. In fact, Parker Stewart took only half the prescribed dose and still died from opioid overdose. We need to prevent harm for anyone taking opioids, including those taking prescription opioids as directed by their healthcare professional for chronic pain or for postoperative or acute pain. We have an obligation to drive education and innovation to eliminate preventable deaths."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.10-13 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,14 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.15 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Opioid Overdose. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose.
- BMJ Newsroom (2020). Vastly differing opioid prescribing patterns in England even in similarly deprived areas. Available at: https://www.bmj.com/company/newsroom/vastly-differing-opioid-prescribing-patterns-in-england-even-in-similarly-deprived-areas/.
- Prescription Opioid Data. CDC Injury Center. Centers for Disease Control and Prevention.
- Levy N. An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia. 2021;76:520–536.
- National Safety Council. Prescription opioid pain killer public opinion poll. October 2017. https://www.cdc.gov /drugoverdose / deaths/ prescription /index.html
- Odds of Dying - Data Details. National Safety Council; Injury Facts, 4 Mar. 2021, injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/data-details.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo SafetyNet Alert™ Launches in Western Europe
Breakthrough Noninvasive Blood Oxygen Monitoring and Alert System Helps Safeguard Patients Taking Opioids at Home
Neuchatel, Switzerland – August 30, 2021 - Masimo (NASDAQ: MASI) announced today the CE marking and launch in Western Europe of Masimo SafetyNet Alert™, an arterial blood oxygen saturation monitoring and alert system designed for use at home. Masimo SafetyNet Alert features Signal Extraction Technology® wearable fingertip pulse oximetry sensor that communicates wirelessly to an accompanying Home Medical Hub and smartphone app. Masimo SafetyNet Alert monitors blood oxygen saturation (SpO2) and pulse rate (PR) using clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry and perfusion index (Pi). The system provides escalating alerts when drops in oxygen levels are detected, designed to wake up the person suffering from opioid overdose and if they do not, to send alerts to others when help may be needed.

Masimo SafetyNet Alert™
Over 200 million people are monitored with Masimo SET® pulse oximetry in hospitals each year.1 In hospitals, continuous Masimo SET® oxygen saturation monitoring has been shown to reduce harm associated with opioids in multiple clinical trials, including a 10-year study in which researchers at Dartmouth-Hitchcock Medical Center found that the use of Masimo SET®-based continuous patient surveillance monitoring resulted in zero opioid-related preventable deaths or brain damage in their post-surgical wards. The researchers also found there was a reduction in rapid rescue events by 60%, a reduction in ICU transfers by 50%, and an estimated $7 million annually in cost savings.2-4
Opioids are powerful painkillers, and are commonly used as part of recovery after surgery and for patients with chronic pain, but they can also slow or stop one’s breathing, potentially leading to heart attack, brain damage, and even death. In 2020 the number of drug-related deaths recorded in England and Wales rose to 4,561, the highest since records began, and around half of these involved opioids.5 Worldwide, that number is even worse, with an estimated more than 100,000 people dying from opioid overdose each year.6 Whether taking prescription or non-prescription opioids, people can suffer from the condition known as opioid-induced respiratory depression (OIRD) to varying degrees.6 Opioid overdose may occur while a person is particularly vulnerable, while asleep, and the risk of opioid overdose-related death is heightened for people taking opioids for the first time, those who have sleep apnea, COPD, or asthma, along with those who combine opioids with alcohol or other sedatives, among other factors.6-8 By monitoring a person’s oxygen saturation level, especially while asleep, and providing escalating alerts when help may be needed, Masimo SafetyNet Alert can help identify life-threatening opioid overdose before it causes lasting harm or even death.
Masimo SafetyNet Alert leverages the same SET® pulse oximetry technology and a similar notification escalation policy used in hospitals to bring hospital-proven monitoring to the home setting. The system provides escalating visual and audible alerts on the smartphone app and at the Home Medical Hub station, which are designed to alert the patient or anyone nearby to help prompt action. If oxygen levels continue to decline, designated emergency contacts, such as friends and family members, are also notified via text messages, so that they can intervene or involve Emergency Medical Services as needed.
Masimo SafetyNet Alert brings to the home the breakthrough Masimo SET® pulse oximetry used in hospitals around the world. SET® has been clinically proven to help care teams enhance patient safety and improve patient outcomes; in fact, more than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies during motion and low perfusion conditions, providing clinicians with increased sensitivity and specificity to make critical care decisions.9
Dr. Mike Durkin, a Senior NHS Advisor on Patient Safety Policy and Leadership for the National Institute for Health Research (NIHR) Imperial College Patient Safety Translational Research Centre, said, "Patients recovering from surgery still need pain relief using opioid drugs after they are discharged from hospital and return home. However, these drugs have significant side-effects, particularly on the depression of breathing, which without urgent intervention can result in serious harm or death. The technology is now available to monitor the impact of opioids on breathing and it is vital that patients are given the opportunity to easily and continuously monitor their oxygen levels and vital signs while taking these medications at home. This will greatly improve the safety of patients while rehabilitating at home."
Yvonne Gardner, mother of 21-year-old Parker Stewart, who died of an opioid overdose after taking only half of the prescribed dose of painkillers following a tonsillectomy, said, "I've had so many people call me personally and say, what would you do differently? My son needs a tonsillectomy, or my daughter is going into surgery. I tell them: make sure your doctor gives you a monitor."
Joe Kiani, Founder and CEO of Masimo, said, "30 years ago, we had the dream of improving patient outcomes and reducing the cost of care by taking noninvasive monitoring to new sites and applications. Bringing our measure-through motion and low perfusion pulse oximetry to the home to monitor patients taking opioids is fulfilling that dream in a way that I could not have imagined at the time. I hope tens of thousands of lives will be saved each year from opioid overdose with the launch of Masimo SafetyNet Alert."
Masimo SafetyNet Alert has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.9 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,10 improve CCHD screening in newborns,11 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.2-4,12 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,1 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.13 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Office for National Statistics. (2021) Deaths related to drug poisoning in England and Wales: 2020 registrations. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity
/birthsdeathsandmarriages/
deaths/bulletins/
deathsrelatedtodrugpoisoninginenglandandwales/2020. Last accessed August 2021. - Opioid Overdose. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose.
- Gupta K et al. Curr Opin Anaesthesiol. 2018;31(1):110-119.
- Subramani Y et al. Br J Anaesth. 2017;119(5):885-899.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet Alert™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet Alert, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Society for the Advancement of Patient Blood Management (SABM) Publication Recommends Continuous Hemoglobin Monitoring
Esteemed Society Recommends the Use of Continuous Hemoglobin Monitoring as Part of Patient Blood Management to Improve Patient Outcomes
Irvine, California – August 26, 2021 - Masimo (NASDAQ: MASI)announced today the conclusions of a whitepaper published by the Society for the Advancement of Patient Blood Management (SABM) which highlights the importance of continuous hemoglobin (Hgb) monitoring to improve patient outcomes in critical care and perioperative settings.1 The authors of the publication concluded that "continuous Hgb monitoring devices provide highly valuable real-time trending data that can assist clinicians in making timelier decisions."
Founded in 2001, SABM is recognized as a key educational resource for Patient Blood Management (PBM). The society defines PBM as the timely application of evidence-based medical and surgical concepts designed to maintain Hgb concentration, optimize hemostasis and minimize blood loss in an effort to improve patient outcomes. To that end, the SABM whitepaper reviews over 10 years of peer-reviewed publications on continuous hemoglobin in making its conclusion. The whitepaper notes that “having continuous access to Hgb levels in real-time offers a clear advantage over the traditional measurement methods as it enables the clinicians to detect changes in Hgb levels quickly and adjust the clinical management strategies accordingly.”
Masimo offers noninvasive and continuous hemoglobin monitoring, SpHb®, as part of its rainbow® Pulse CO-Oximetry platform. To display SpHb trends, Masimo rainbow® sensors can connect to a variety of Masimo Pulse CO-Oximeters®, including patient monitors available or in development from more than 20 other Masimo OEM Partners. For details, visit masimo.com/oem/partners. By utilizing multiple wavelengths of light, SpHb provides real-time visibility to changes, or lack of changes, in hemoglobin between invasive blood samples.
Continuous hemoglobin monitoring with SpHb as part of PBM programs has been found to improve outcomes in both high- and low- blood loss surgeries, such as reducing the percentage of patients receiving transfusions,2 reducing the units of red blood cells transfused per patient,3-4 reducing the time to transfusion,5 reducing costs,6 and even reducing mortality 30 and 90 days after surgery by 33% and 29%, respectively.7 This evidence of SpHb's impact on outcomes spans the globe, representing 6 countries on 4 different continents.2-8
One of the key references cited by the whitepaper is the recent SABM Administrative and Clinical Standards for PBM Programs (5th edition). These standards recommend the "use of noninvasive hemoglobin and other laboratory measurements" as part of a strategy to reduce phlebotomy blood loss.9 Similarly, the latest pediatric standards from SABM (4th edition) recommend that "noninvasive techniques are used for monitoring of blood gases, hemoglobin and other analytes whenever possible."10
Sherri Ozawa, RN, President of SABM, commented, "We believe that comprehensive patient blood management will be the global standard of care. The evidence shows that patient outcomes are better when a patient's own blood is well managed and unnecessary transfusions are avoided. Clinicians need access to timely information that allows them to make better informed decisions, such as the information provided by noninvasive hemoglobin technology."
The SABM whitepaper also discusses the role of pleth variability index (PVi®) as part of PBM. PVi is a continuous, noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients. It is a measure of the dynamic changes in perfusion index that occur during the respiratory cycle. The authors note that "combining such a measure with noninvasive Hgb monitoring can yield a more comprehensive picture of the hemodynamic and circulatory status of the patients." More than 100 independent studies have demonstrated the utility of PVi as an indicator of fluid responsiveness.11
Dr. William Wilson, Chief Medical Officer of Masimo, stated, "Anesthesiologists and critical care physicians have long recognized the importance of dynamic measures of intravascular volume and fluid responsiveness. With the combination of PVi and SpHb, clinicians have a wealth of information at the bedside accessible through something as ubiquitous as a pulse oximetry sensor."
Joe Kiani, Founder and CEO of Masimo, commented, "Since the launch of SpHb in 2008, we have seen over 75 countries adopt this technology and numerous publications noting its positive impact on outcomes. SABM is recognized as the premier provider of professional education in patient blood management. It's great to see SABM recommend the use of SpHb and PVi to help improve patient outcomes."
***
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgement considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
The accuracy of PVi in predicting fluid responsiveness is variable and influenced by numerous patient, procedure, and device-related factors. PVi measures the variation in the plethysmography amplitude but does not provide measurements of stroke volume or cardiac output. Fluid management decisions should be based on a complete assessment of the patient’s condition and should not be based solely on PVi.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.12 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,13 improve CCHD screening in newborns,14 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.15-18 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,19 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.20 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Society for the Advancement of Blood Management (SABM). Improvement of Patient Outcomes with Hemoglobin Monitoring in the Critical Care and Perioperative Setting. Publications. 2021, January. https://sabm.org/wp-content/uploads/SABM-HbMonitoringWhitepaper.pdf
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Merolle L, Marraccini C, Di Bartolomeo E, Montella M, Pertinhez T, Baricchi R, Bonini A. Postoperative patient blood management: transfusion appropriateness in cancer patients. Blood Transfus 2020; 18: 359-65 DOI 10.2450/2020.0048-20.
- Society for the Advancement of Blood Management (SABM). SABM Administrative and Clinical Standards for Patient Blood Management Programs 5th Edition. Publications. 2019. https://sabm.org/wp-content/uploads/SABM-Standards-20196.pdf
- Goobie SM, Gallagher T, Gross I, Shander A. Society for the advancement of blood management administrative and clinical standards for patient blood management programs. 4th edition (pediatric version). Paediatr Anaesth. 2019 Mar;29(3):231-236. doi: 10.1111/pan.13574. PMID: 30609198.
- Published clinical studies on PVi, with varying results and outcomes, can be found on our website at http://www.masimo.com/evidence/pulse-oximetry/pvi. Studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb® and PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb and PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Evaluates the Use of Masimo PVi® to Monitor Volume Status in Spontaneously Breathing Hemodialysis Patients
Neuchatel, Switzerland – August 23, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the <em>Turkish Journal of Emergency Medicine</em> in which Drs. Seda Dağar and Hüseyin Uzunosmanoğlu at the Kecioren Training and Research Hospital in Ankara, Turkey investigated the role that noninvasive, continuous Masimo PVi<sup>®</sup> might play in monitoring volume status and volume changes in spontaneously breathing patients undergoing hemodialysis (HD).<sup>1</sup> The researchers found that there was a "strong correlation" between change in PVi and the volume of fluid removed, concluding that "PVi may provide clinicians with useful information for monitoring the volume status in critically ill patients with spontaneous breathing."
More than 100 independent studies have demonstrated the utility of PVi as an indicator of fluid responsiveness.2 Noting that PVi has been studied mostly in mechanically ventilated patients, the researchers sought to investigate its ability to help assess volume changes in spontaneously breathing patients. They enrolled 60 adult patients with end-stage renal disease who received routine HD (during which fluid is removed simultaneously with the removal of waste solutes) and had a median of 3,500 cc of fluid removed during HD. PVi was measured using a Masimo pulse oximetry sensor attached to a Masimo Root® monitor, before and after HD, and changes in PVi were compared to the amount of fluid removed during the session.
The researchers found that mean PVi showed a statistically significant increase after HD, from 20.7% ± 5% to 27.7% ± 6% (p < 0.001). Based on the amount of fluid removed during HD, the change in PVi was statistically significant (p = 0.015) and was strongly correlated to the volume of fluid removed (r = 0.744, p < 0.001).
The researchers concluded, "In the present study, we found that the fluid removed by HD in spontaneously breathing patients caused an increase in PVi and that this increase was strongly correlated with the amount of volume change. Bedside monitoring of PVi, which is a noninvasive, fast, reproducible measurement parameter, may provide the clinicians with useful information for monitoring the volume status and evaluating the effectiveness of volume‑restoration therapy in critically ill patients with spontaneous breathing."
The accuracy of PVi in predicting fluid responsiveness is variable and influenced by numerous patient, procedure, and device-related factors. PVi measures the variation in the plethysmography amplitude but does not provide measurements of stroke volume or cardiac output. Fluid management decisions should be based on a complete assessment of the patient’s condition and should not be based solely on PVi.
In the U.S., PVi is cleared as a noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Dağar S, Uzunosmanoğlu H. Assessment of pleth variability index in volume changes during ultrafiltration process. Turkish Journal of Emergency Medicine. 2021;21:111-6.
- Published clinical studies on PVi, with varying results and outcomes, can be found on our website at http://www.masimo.com/evidence/pulse-oximetry/pvi. Studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Evaluates the Use of Masimo Bridge® As an Alternative to Opioids
Irvine, California – August 16, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the Journal of Pain & Relief in which Dr. Jacques Chelly and colleagues at the University of Pittsburgh investigated the utility of Masimo Bridge®, an auricular field nerve stimulator, in reducing postoperative opioid requirements in patients undergoing kidney donor surgery.1 The researchers found that use of Bridge was associated with significant reductions in opioid use at 24 hours and pain at 24 and 48 hours after surgery, concluding that Bridge "may represent a complementary approach to minimize the postoperative requirement for opioid[s]."
Noting that opioids are still considered to be required after kidney donor surgery, in spite of their "established serious side effects including respiratory depression, postoperative nausea and vomiting" and that they "expose the donors to an unnecessary risk of opioid use disorder," the researchers sought to evaluate whether the technique of percutaneous nerve field stimulation might provide benefit during the postoperative period. To evaluate this, they enrolled 20 patients undergoing kidney donor surgery, divided into two groups of 10. Both groups underwent the same enhanced recovery after surgery (ERAS) protocol based on a multimodal analgesic approach. The primary endpoint was opioid requirement, measured as oral morphine equivalent (OME), 24 hours after surgery. They also evaluated pain (on a 0 to 10 scale) 24 and 48 hours after surgery. In the experimental group (n=10), Masimo Bridge was fitted on patients in the post-anesthesia care unit.
The researchers found that, compared to the control group (n=10), the patients in the Bridge group had a 75.4% reduction in OME (8.3 ± 9.6 mg vs. 33.5 ± 37.3 mg, p = 0.03) and a 58.3% reduction in pain (2.5 ± 2.0 vs. 6 ± 1.4, p < 0.001) at 24 hours. At 48 hours, they had a 16.1% reduction in OME (22.4 ± 19.5 mg vs. 26.7 ± 21.9 mg, p = 0.33) and a 73.3% reduction in pain (1.6 ± 1.6 vs. 6.0 ± 2.8, p = 0.0004). There was no difference in non-opioid analgesic use between the two groups. "Tolerability" of the Bridge device was reported as "excellent" by 78% of participants.
The researchers concluded, "This study suggests that the NSS-2 [Masimo] Bridge device may be of significant value in controlling postoperative opioid consumption and pain. This is especially interesting in the context of the current opioid epidemic and associated risk of opioid use disorder (OUD) in surgical patients. However, a prospective randomized placebo control design is required to confirm our findings."
Masimo Bridge, the first evidence-based, drug-free, non-surgical device of its kind, has been cleared by the FDA for use in the treatment of clinical symptoms associated with opioid withdrawal, but is not currently FDA-cleared for postoperative pain management. The solution consists of a wearable, single-patient-use, percutaneous neurostimulator, fitted behind the ear, which applies gentle electrical impulses to branches of the cranial nerves around the ear. By using neuromodulation to aid in the reduction of withdrawal symptoms, Bridge may help patients with OUD successfully transition into a treatment program. In clinical testing, Bridge was found to reduce opioid withdrawal symptoms within 15-30 minutes and provide continuous relief for as long as it was applied, which can be up to 120 hours per device, allowing opioids to leave the body. In a study of 73 adults with OUD, it was shown that opioid withdrawal symptoms (such as increases in resting pulse rate, sweating, restlessness, bone or joint aches, tremors, and anxiety) were reduced by 85% after the first hour of using the device and 97% after 5 days of use (measured using the clinical opiate withdrawal scale).2
Study author Dr. Chelly commented, "Auriculotherapy is an ancient technique that has been used for centuries to treat pain. However, it requires long and specialized training and experience to be used effectively on patients. The Bridge device, by contrast, provides similar therapy but requires only limited training. Bridge offers the possibility of allowing many more patients to benefit from auriculotherapy, especially at a time when the use of opioids is so controversial."
Joe Kiani, Founder and CEO of Masimo, said, "With FDA clearance, we have been marketing Bridge for opioid withdrawal for over a year and have seen amazing success stories. With this new promising study from UPMC, we will now work towards getting Bridge cleared for postoperative pain treatment worldwide. Last year, over 83,000 people died in the US alone from drug overdoses, with almost 63,000 of them killed by opioids, many of them from prescription opioids used post surgery.3 While we hope that with our FDA-pending, breakthrough-designated Masimo SafetyNet™ opioid solution, we will catch opioid overdoses early enough to save lives, it would be best if we can avoid the unnecessary use of opioids altogether. We hope additional studies will confirm that Bridge can help reduce the use of opioids."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Monroe AL, Planinsic RM, Tevar A, Chelly JE. Auricular field nerve stimulation using the NSS-2 Bridge® device as an alternative to opioids following kidney donor surgery. J Pain Relief. 2021. 10:365.
- Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am J Drug Alcohol Abuse. 2017 Mar 16;1-8.
- Provisional Drug Overdoes Death Counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Bridge® and Masimo's plan to obtain additional FDA clearance for Masimo Bridge. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks that a prospective randomized placebo control design is unable to confirm the findings in the new study from UPMC; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Bridge, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks of not gaining FDA clearance or marketing approvals for its product candidates, including Masimo Bridge; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Introduces the MX-7™ rainbow SET® Technology Board for Original Equipment Manufacturing Partners
Irvine, California – July 19, 2021 - Masimo (NASDAQ: MASI) announced today the release of MX-7™, its latest and most advanced rainbow SET® board. Designed for integration into the more than 200 multi-parameter monitors available from its more than 90 original equipment manufacturing (OEM) partners, MX-7 has the ability to support all 13 of Masimo's SET® pulse oximetry and rainbow® Pulse CO-Oximetry measurements in an advanced module re-engineered to reduce power needs.
The MX-7 adds to Masimo's growing portfolio of technology boards used both in its own patient monitors and also available to its OEM partners. MX-7 builds on the current MSX™ low-power SET® board and MX-5™ rainbow® board by offering more efficient power utilization, scaling its power draw based upon the combination of rainbow SET® parameters being monitored to permit even longer battery run times.
The MX-7 offers the full suite of Masimo's advanced noninvasive SET® and rainbow® parameters. Masimo SET® pulse oximetry has been clinically proven to help care teams enhance patient safety and improve patient outcomes; in fact, more than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies during motion and low perfusion conditions, providing clinicians with increased sensitivity and specificity to make critical care decisions.1 SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce deaths due to opioid overdose and reduce rapid response team activations, ICU transfers, and costs.4-7
By leveraging additional wavelengths of light and breakthrough signal processing, Masimo rainbow® technology allows clinicians to noninvasively and continuously monitor multiple advanced parameters that previously could only be measured using invasive techniques.
Among other rainbow SET® parameters, MX-7 offers:
- Masimo SET® SpO2 (oxygen saturation) and pulse rate (PR).
- Noninvasive and continuous hemoglobin monitoring, SpHb®, which offers real-time visibility to changes, or lack of changes, in hemoglobin between invasive samples. Continuous monitoring with SpHb monitoring as part of patient blood management (PBM) programs has been found to improve numerous outcomes.8-14
- Pleth Variability Index (PVi®), an index of continuous, noninvasive, dynamic indication of fluid responsiveness in select populations of mechanically ventilated adult patients.
- SpMet®, which allows clinicians to noninvasively and continuously monitor levels of methemoglobin in the blood.
- SpCO®, a noninvasive and continuous measurement of the carbon monoxide levels in arterial blood.
- SpMet®, which allows clinicians to noninvasively and continuously monitor levels of methemoglobin in the blood.
- Acoustic respiration rate (RRa®), which uses acoustic signal processing to provide noninvasive and continuous monitoring of respiration rate for all patient populations.
- Plethysmographic respiration rate (RRp®), which allows clinicians to seamlessly implement monitoring of this key vital sign without additional equipment by using the same SET® pulse oximetry sensor that monitors oxygen saturation (SpO2).
- Oxygen Reserve Index (ORi™), a noninvasive and continuous parameter intended to provide additional insight into a patient’s oxygen status under supplemental oxygen.
Joe Kiani, Founder and CEO of Masimo, said, "The MX-7 board represents that ongoing drive for innovation and improvement, making sure that our full suite of cutting edge SET® and rainbow® measurements are universally available to all of our customers."
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,15 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.16 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Merolle L et al. Postoperative patient blood management: transfusion appropriateness in cancer patients. Blood Transfus 2020; 18: 359-65 DOI 10.2450/2020.0048-20.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MX-7™ and rainbow SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MX-7 and rainbow SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Evaluates the Ability of Masimo SedLine® Brain Function Monitoring to Predict Neurological Outcomes and Long-term Survival in Post-Cardiac Arrest ICU Patients
Researchers Found That the Combination of Two SedLine Parameters, Patient State Index (PSi) and Suppression Ratio (SR), Had High Predictability for Mortality 180 Days After Cardiac Arrest
Neuchatel, Switzerland – July 6, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the Journal of Critical Care in which Dr. Tae Youn Kim and colleagues at the Dongguk University College of Medicine and Yonsei University College of Medicine in Korea evaluated the ability of two parameters provided by Masimo SedLine® brain function monitoring to predict neurological outcomes and long-term survival in post-cardiac arrest ICU patients.1 The researchers found that the combination of the two parameters, Patient State Index (PSi) and Suppression Ratio (SR), had “high predictability” for mortality 180 days after cardiac arrest.
Noting that “accurate prognostication” in post-cardiac arrest patients is important to determine treatment plans and “whether to continue or withdraw intensive care,” and that a “multi-modal” approach is recommended because “no single prognostic factor has been shown to have higher prognostic accuracy than those of other factors,” the researchers sought to evaluate the prognostic accuracy of the two Masimo SedLine parameters as predictors of neurological outcomes, both alone and in combination. The researchers chose PSi because, as they note, raw EEG data can be “difficult to use” and PSi, which is derived from EEG, is widely used in anesthesiology for determining the degree of procedural sedation, “significantly co-varies with changes in the state” under general anesthesia, and “can significantly predict” the level of arousal in varying stages of anesthetic delivery. The researchers chose SR because it helps to estimate the percentage of EEG suppression and is therefore considered a good predictor of poor neurologic outcomes.2
They enrolled 103 adult patients between January 2017 and August 2020 who experienced a non-traumatic out-of-hospital cardiac arrest, had been successfully resuscitated after CPR, and received targeted temperature management during their ICU stay. PSi and SR were continuously monitored using Masimo SedLine from immediately after ICU admission until 24 hours after return of spontaneous circulation (ROSC), recorded at one hour intervals. Neurological outcomes were categorized using the Pittsburgh Brain Stem Score (PBSS) and Cerebral Performance Category (CPC). Data on survival at 180 days was obtained via telephonic interviews.
The researchers found that using either PSi or SR alone had “good predictability” for poor neurological outcome, and that the combination of low PSi and SR had “high predictability” for mortality 180 days after cardiac arrest. They used receiver operating characteristic (ROC) curves to determine that “a mean PSI ≤ 14.53 and mean SR > 36.6 showed high diagnostic accuracy” as single prognostic factors for patients in their study. Furthermore, “Multimodal prediction using the mean PSi and mean SR showed the highest area-under-the-curve value of 0.965 (95% confidence interval 0.909–0.991).” In the study cohort, patients with mean PSi ≤ 14.53 and mean SR > 36.6 had “relatively higher long-term mortality rates” (69% died in the group) than those of patients with values > 14.53 and ≤ 36.6 (11% died in the group).
The researchers concluded that “PSi and SR are good predictors for early neuro-prognostication in post-cardiac arrest patients.” They also noted, “The combination of PSI and SR showed better predictability of poor neurologic outcome than did each individual parameter.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Kim TY, Hwang SO, Jung WJ, et al. Early neuroprognostication with the Patient State Index and suppression ratio in post-cardiac arrest patients. J Crit Care. 2018. https://doi.org/10.1016/j.jcrc.2021.06.003.
- Seder DB, Fraser GL, Robbins T, Libby L, Riker RR. The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med 2010;36(2):281-8.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces FDA 510(k) Clearance of Radius T°™ for Prescription and Over-the-Counter (OTC) Use
The Radius T° Wearable Continuous Body Temperature Thermometer Is Now Available for Use in Hospital and at Home
Irvine, California – June 21, 2021 - Masimo announced today that Radius T°™, a wearable, wireless thermometer that measures body temperature continuously and noninvasively, has received FDA 510(k) clearance for both prescription and over-the-counter (OTC) use on patients and consumers five years and older. Featuring continuously trending temperature measurements and Bluetooth® connectivity, Radius T° automates remote, continuous body temperature status for clinicians through its paired connection to a Masimo patient monitoring or telehealth solution, and for consumers through the Masimo Radius T° smartphone application. Radius T° is part of the growing family of tetherless Masimo technologies that includes Radius PPG™, which offers Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, and Radius PCG™, which provides mainstream capnography.
For prescription use, Radius T° is compatible with both the Root® Patient Monitoring and Connectivity Hub and the Rad-97® patient monitor for use in the hospital, as part of the Masimo Hospital Automation™ platform. Radius T° can also be paired to a patient's smartphone using the Masimo SafetyNet™ app, for remote patient management from the home or other care location. The wireless thermometer is designed to help streamline workflows, with its convenient wearability, continuous measurements, and wireless connectivity, reducing the need for intermittent manual temperature checks with traditional probes that require a clinician's presence at the bedside. Each Radius T° wireless thermometer is single-patient use, to reduce the risk of cross-contamination between patients, with an 8-day battery life and shower-proof adhesive, reducing the need to remove or replace the sensor – thus supporting a hospital's or caregiver's temperature monitoring and infection control workflow.
Using a proprietary algorithm, Radius T° provides continuous body temperature measurements that are approximations of sublingual temperatures captured from an oral probe. Radius T° supports continuous body temperature performance that goes beyond simple skin surface temperature measurements, and remains reliable even when environmental temperatures are fluctuating. Radius T° provides trended data to help clinicians manage and review a patient’s high fever risks and fever progress over time.
Radius T° is easily integrated into the Masimo Hospital Automation platform, through Root or Rad-97, allowing automatic transfer of continuous body temperature data to a hospital's electronic medical records (EMRs) or surveillance monitoring solution, thus streamlining clinicians' ability to gain a more insightful picture of each patient’s physiological status, regardless of the clinician’s physical location.
For consumers, Radius T° represents a paradigm shift in thermometry by making it continuous, wearable, and hassle-free. Traditional periodic and invasive methods depend on the user repeatedly conducting a series of steps that can interrupt daily activities, including sleep, and can miss body temperature trends and patterns. With a traditional thermometer, a person may only notice a spike in temperature hours after a spike has occurred, or may not even become aware of it if it is during sleep. By contrast, Radius T° continuously measures temperatures and seamlessly transmits data and customizable temperature notifications to the Masimo Radius T° app on the user's smartphone – helping caregivers, such as parents, monitor loved ones' temperatures even while they sleep and providing continuous insight into changes and trends in their temperature. In addition, Radius T° is applied comfortably on the upper chest, avoiding the potential discomfort of other wearable devices that rely on placement in the armpit.
Joe Kiani, Founder and CEO of Masimo, said, "Radius T° was first made available in the U.S. under an FDA COVID-19 enforcement policy for thermometers as part of Masimo SafetyNet – where it quickly demonstrated its value in improving clinician workflows through its continuous, remotely accessible body temperature measurements for patients recovering or quarantined at home. Now, with the FDA 510(k) clearance, the availability of the Radius T° is being expanded for use by both clinicians and consumers to help monitor and track body temperatures in all thermometer applications."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radius T°™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radius T°, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Investigates the Impact of ORi™-Guided Oxygen Titration on Hyperoxemia-Mediated Morbidity During One-Lung Ventilation
Researchers Found That ORi-Guided Thoracic Anesthesia May Reduce Hospital Length of Stay and Increase Patient Safety
Neuchatel, Switzerland – June 14, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the Journal of PeriAnesthesia Nursing in which Drs. Mashasi Ishikawa and Atsuhiro Sakamoto at Nippon Medical School in Tokyo evaluated the utility and impact of Masimo Patient SafetyNet™ by surveying nurses before and after implementation.1 The researchers found that use of the remote monitoring and clinical notification system decreased the number of physical assessments needed, resulting in a reduction in the nursing workload, and also recommended the use of continuous respiratory rate and oxygen saturation monitoring (which was implemented as part of the system) after general anesthesia for patients' safety.
ORi, available outside the U.S. since 2014, is a noninvasive and continuous parameter intended to provide additional insight into a patient's oxygen status under supplemental oxygen. Enabled by the multi-wavelength rainbow® Pulse CO-Oximetry platform, ORi is provided alongside oxygen saturation (SpO2) measured by clinically proven Masimo SET® pulse oximetry.
Noting that during OLV, a common technique for facilitating a wide variety of pulmonary procedures, 100% fraction of inspired oxygen (FiO2) supplemental oxygen administration is commonly used, which exposes patients to the possibility of hyperoxia-induced lung injury, the researchers sought to determine whether ORi could "protect patient from the harmful effects of hyperoxemia with a noninvasive probe during OLV." They divided 103 patients with lung tumors (18-70 years of age, ASA I-III), enrolled between September 2018 and September 2019 and requiring OLV as part of elective thoracic surgery, into four groups as noted below:
|
Group |
Number of Subjects |
Duration of OLV with FiO2 > 60% |
Mean FiO2 Values |
|
1: Oxygen titration without ORi, low-flow anesthesia (1 L/min) |
25 |
67.6 ± 97.5 mins. |
71.6 ± 12.25% |
|
2: Oxygen titration without ORi, high-flow anesthesia (4 L/min) |
28 |
97.32 ± 99.7 mins. |
74.64 ± 16.66% |
|
3: Oxygen titration with ORi, low-flow anesthesia (1 L/min) |
25 |
39.2 ± 74.1 mins. |
62.8 ± 13.08% |
|
4: Oxygen titration with ORi, high-flow anesthesia (4 L/min) |
25 |
22.4 ± 49.4 mins. |
56.4 ± 11.5% |
During OLV, oxygen titration was performed without ORi for patients in groups 1 and 2, while groups 3 and 4 used ORi from Masimo Radical-7® Pulse CO-Oximeters® and rainbow® sensors. For all 4 groups, SpO2 and partial pressure of arterial oxygen (PaO2) were routinely measured while FiO2 was routinely administered at 50% after induction, rising to 60% when the OLV was applied, and increasing to 70%-100% as necessary.
The researchers found that ORi monitoring was associated with significantly shorter duration of OLV with FiO2 > 60%, with significantly lower mean FiO2 values during OLV (as shown in the table above), and with lower recorded PaO2 values. They also found that the duration of FiO2 > 80% during OLV was strongly correlated with longer hospital stays (p < 0.001).
The observation that mean FiO2 values were found to be significantly lower in the ORi groups (3 and 4), compared to the groups without ORi (1 and 2), led the researchers to hypothesize that "the risk of hyperoxia will be lower in patients undergoing ORi monitor[ing]." The researchers noted, "ORi cannot replace arterial blood gas analysis; however, it is useful to assess oxygenation. In groups without ORi monitors, the FiO2 was significantly higher than 80%. Moreover, in our study, it was revealed that these patients had a longer hospital stay."
There was no significant difference in ORi values between the low-flow (group 3) and high-flow (group 4) cohorts.
The researchers concluded, "The adjustment of ORi with peripheral oxygen saturation and blood gas analysis demonstrated that hyperoxemia could be prevented during OLV in patients under low flow or high flow anesthesia. We concluded that ORi-guided thoracic anesthesia may reduce hospital stay and increase patient safety."
ORi has not yet received FDA clearance and is not available in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Saracoglu A, Yamansavci Şirzai E, Yildizeli B, Yüksel M, Aykaç ZZ. Oxygen Reserve Index Guided Oxygen Titration in One Lung Ventilation with Low Fresh Gas Glow. Turkish Journal of Medical Sciences. 2021 May. DOI: 10.3906/sag-2009-149.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Assesses the Effects of Masimo Patient SafetyNet™ on Nursing Workflows in the General Ward
Researchers Found That Use of Patient SafetyNet with Masimo SET® Pulse Oximetry and Acoustic Respiratory Rate (RRa®) Monitoring Reduced Nursing Workload Related to Postoperative Respiratory Assessment by More Than 60%
Neuchatel, Switzerland – May 17, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the Journal of PeriAnesthesia Nursing in which Drs. Mashasi Ishikawa and Atsuhiro Sakamoto at Nippon Medical School in Tokyo evaluated the utility and impact of Masimo Patient SafetyNet™ by surveying nurses before and after implementation.1 The researchers found that use of the remote monitoring and clinical notification system decreased the number of physical assessments needed, resulting in a reduction in the nursing workload, and also recommended the use of continuous respiratory rate and oxygen saturation monitoring (which was implemented as part of the system) after general anesthesia for patients' safety.
Noting the importance of frequent postoperative respiratory assessment, especially for patients on opioids, the researchers hypothesized that use of Patient SafetyNet, which displays near real-time information from connected bedside patient monitors at central/remote surveillance stations, could facilitate such evaluations "without major patient complications." To study the effects of adopting such a solution, they implemented Masimo Hospital Automation™ with Patient SafetyNet and Masimo Radical-7® Pulse CO-Oximeters® at the bedside, on all general floors. After implementation, patients' oxygen saturation (SpO2) and acoustic respiration rate (RRa®) were continuously monitored at the bedside, with the data relayed to the central Patient SafetyNet View Stations. Remote alarm notifications were programmed for the following conditions: SpO2: < 90% for > 10 seconds; bradypnea: < 8 breaths/minute for > 2 minutes; tachypnea: > 30 breaths/minute for > 2 minutes. When any of these physiological limits was violated, nurses performed a manual respiratory check (which typically involved use of a stethoscope and a pulse oximeter).
To measure the impact of the Patient SafetyNet system with continuous acoustic respiration rate monitoring, the researchers surveyed 75 nurses 3 months before and 1 month after implementation, asking about a variety of methods and problems related to postoperative respiratory monitoring before/after use of the system; the usefulness of a central/remote monitoring system; and the effects of Patient SafetyNet on their workload. Among other results, the percentage of nurses who found central remote monitoring to be useful increased from 78.7% pre-implementation to 89.3% post-implementation, and the percentage who found continuous monitoring useful increased from 88.0% to 98.7%. 96% of nurses reported that they were able to attend patient bedsides within one minute of alarm occurrence. Problems recorded in the surveys included false alarms related to tachypnea, triggered by the patient's speaking, and a tendency to avoid early ambulation because of being continuous monitored.
In addition, the researchers collected retrospective data from patient records about the number of postoperative respiratory checks each patient received for 3 months before and 3 months after system implementation. They found that the average frequency of clinical examination was reduced from 11.0 ± 2.3 to 5.1 ± 1.3, representing a reduction of 61.3% in nursing workload related to postoperative respiratory assessment.
The researchers concluded, "The merits of the Patient SafetyNet system were that it could be useful for early detection when the respiratory condition gets worse and evaluation of the causes for deterioration of respiratory status using numerical values and waveforms. Therefore, the Patient SafetyNet system is suitable for cases requiring continuous sedative or opioid infusions, with poor general condition or depressed levels of consciousness. Continuous monitoring of respiratory rate and SpO2 after general anesthesia is recommended for patients' safety. Moreover, the Patient SafetyNet system can decrease the number of respiratory assessments of postoperative patients in the general wards, resulting in reduction of nurse’s workload."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Ishikawa M, Sakamoto A. Patient SafetyNet for the Evaluation of Postoperative Respiratory Status by Nurses: A Presurvey and Postsurvey Study. J PeriAnesth Nursing. DOI: https://doi.org/10.1016/j.jopan.2020.03.005.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™, Radical-7®, SET®, and RRa®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, Radical-7, SET®, and RRa, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Study Investigates the Ability of Masimo O3® to Aid in Monitoring Septic Shock ICU Patients and Predicting Mortality
Researchers Found That O3 May Help Clinicians Rapidly Assess Circulatory Status in Patients Experiencing Septic Shock and Have Prognostic Value in Mortality Prediction
Neuchatel, Switzerland – May 10, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the Journal of Anesthesia & Clinical Research in which Dr. Debdipta Das and colleagues at Medical College Kolkata in India evaluated the utility of Masimo O3® Regional Oximetry to aid in monitoring septic shock patients admitted to the ICU.1 O3, available on the Masimo Root® Patient Monitoring and Connectivity Platform, uses noninvasive near-infrared spectroscopy (NIRS) to enable monitoring of regional oxygen saturation (rSO2) in the region of interest, such as the brain or cerebral tissue (CrSO2). The researchers found that O3 trends correlated significantly with a variety of other hemodynamic variables and lactic acid levels commonly used in the monitoring of septic shock patients. They also found rSO2 values differed significantly between patients who survived and those who did not, suggesting it may have value as a predictor of mortality in septic shock patients.
Noting the value of assessing tissue perfusion to aid in the management of hemodynamically challenged ICU patients, the researchers sought to evaluate whether noninvasive cerebral regional oxygen saturation monitoring might prove a viable adjunct or alternative to methods that may be affected by the patient’s baseline condition (such as vital signs monitoring) and invasive methods (like blood lactate measurement), which carry a host of limitations (intermittent and delayed measurement, blood loss, etc.). To do so, they monitored 40 adult patients, diagnosed with septic shock and admitted to the Critical Care Unit, with Masimo O3 and a variety of other measurements every six hours for 72 hours after admission.
The researchers found that O3 cerebral oxygenation trends correlated significantly with other parameters commonly used in monitoring septic shock patients. There was a significant negative correlation between cerebral rSO2 and lactic acid (r = -0.749 to -0.956) after the first six hours after admission. They also found significant positive correlations between cerebral rSO2 and central venous saturation (r = 0.904 to 0.993), mean arterial pressure (r = 0.957 to 0.993), and arterial oxygen saturation (r = 0.864 to 0.988).
Notably, the researchers found that survivors (n = 29) had a significant difference in cerebral rSO2 over the 72 hours after admission compared to non-survivors (n = 11) (p < 0.001), suggesting that O3 rSO2 measured on cerebral tissue may have predictive value for mortality in septic shock patients.
As part of the study, patients with cerebrovascular stroke, cerebral infarction, cerebral hemorrhage, convulsions, or paresis, as well as patients with a more than 10% difference in rSO2 values between the two sides of the brain, were excluded.
The researchers concluded that Masimo O3 rSO2 on cerebral tissue "could be [a] parameter in patients with shock and it could have a prognostic value in mortality prediction and clinical outcome." They elaborated, "In the current study, we highlight other new advantages of NIRS monitoring in circulatory shock (septic) as a continuous monitor and as an outcome predictor. The most important observation from this study is the significant correlation between NIRS oximetry readings and hemodynamic variables, especially lactic acid [and] central venous oxygen saturation in patients experiencing shock. These data, therefore, propose a method for rapid and noninvasive assessment of circulatory status in patients experiencing shock."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Das D, Mitra K, Das S. Brain CO-Oximetry: A Useful Noninvasive Parameter Adjuvant to Standard Perfusion Parameters in Septic Shock. J Anesth Clin Res. 12(1)987.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including O3, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Study Investigates the Ability of Masimo PVi® to Predict Preload Responsiveness in Patients On Nasal High-Flow Therapy
Researchers Found That PVi May Be Used to Guide Fluid Administration in Nasal High-Flow Patients
Neuchatel, Switzerland – May 3, 2021 - Masimo (NASDAQ: MASI) announced today the findings of a study published in the Journal of Applied Physiology in which Dr. Marina García-de-Acilu and colleagues at the Vall d’Hebron University Hospital in Barcelona evaluated the utility of Masimo PVi® as a noninvasive method of predicting preload responsiveness in patients treated with nasal high-flow (NHF) therapy. They found that PVi may identify preload responders and noted that PVi may therefore be used in the "day-to-day clinical decision-making process in critically ill patients treated with NHF, helping to provide adequate resuscitation volume."1 More than 100 independent studies have demonstrated the utility of PVi as an indicator of fluid responsiveness.2 This is the first time PVi has been evaluated in patients treated with NHF therapy.
Noting the potential convenience of a noninvasive method of predicting fluid responsiveness in NHF patients, the researchers sought to evaluate whether PVi, which is noninvasive and easy to use, could play such a role. To do so, they compared PVi to reference measurements – stroke volume (SV) and cardiac output (CO) – in 20 adult ICU patients with acute respiratory failure (ARF) supported by NHF (flow ≥ 30 L/min). SV and CO were measured using transthoracic echocardiography (TTE) using a portable echocardiogram. PVi was measured using a Masimo Radical-7® Pulse CO-Oximeter® with a pulse oximetry sensor attached to the finger. Within the first 24 hours of NHF support, the patients' SV/CO and PVi were assessed. Passive leg raising (PLR) was performed and SV/CO and PVi were then reassessed. Preload responsiveness was defined as a ≥ 10% increase in SV after PLR. A fluid challenge was then conducted by administering a 250-mL saline solution to patients who were found to be preload responders (12 of the 20 patients). SV/CO and PVi were measured again after the fluid challenge in these patients.
The researchers found that preload responders showed higher baseline PVi values and ΔPVi after PLR. PVi and ΔPVi after PLR showed "excellent diagnostic accuracy for predicting preload responsiveness." At a baseline cut-off value of 16%, PVi had sensitivity of 91.7% and specificity of 87.8% for discriminating between preload responders and non-responders; a change of 2% or more in PVi allowed for discrimination between the two groups with 100% sensitivity and specificity. Additionally, the researchers found that ΔPVi after PLR and after fluid challenge were strongly correlated (r = 0.84, p < 0.001).
The researchers concluded, "This physiological study suggests that PVi might predict preload responsiveness in hypoxemic ARF patients treated with NHF. Further research should focus on validating these results and analyze whether PVi-guided fluid administration can improve outcomes in NHF patients."
The researchers also noted that PVi may not be sufficient to identify preload responders in all patients using NHF, hypothesizing that the intrathoracic pressures delivered by NHF are lower than those generated during invasive mechanical ventilation and that therefore a certain degree of hypoperfusion might potentially be required to effect changes in baseline PVi.
The accuracy of PVi in predicting fluid responsiveness is variable and influenced by numerous patient, procedure, and device-related factors. PVi measures the variation in the plethysmography amplitude but does not provide measurements of stroke volume or cardiac output. Fluid management decisions should be based on a complete assessment of the patient’s condition and should not be based solely on PVi.
In the U.S., PVi is cleared as a noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- García-de-Acilu M, Pacheco A, Santafé M, Francisco-Javier R, Ruiz-Rodríguez J, Ferrer R, Roca O. Pleth variability index may predict preload responsiveness in patients treated with nasal high flow: a physiological study. J Appl Physiol. 2021 Apr 15. DOI: 10.1152/japplphysiol.00614.2020.
- Published clinical studies on PVi, with varying results and outcomes, can be found on our website at http://www.masimo.com/evidence/pulse-oximetry/pvi. Studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces FDA Clearance of Radius PCG™ for the Root® Patient Monitoring and Connectivity Platform
Radius PCG with Bluetooth® Connectivity Seamlessly Integrates Tetherless Mainstream Capnography with Root
Irvine, California – April 12, 2021 - Masimo (NASDAQ: MASI) announced today that Radius PCG™, a portable real-time capnograph with wireless Bluetooth® connectivity, has received FDA 510(k) clearance. Radius PCG connects with the Root® Patient Monitoring and Connectivity Platform to provide seamless, tetherless mainstream capnography for patients of all ages. Radius PCG joins the growing family of tetherless Masimo technologies that includes Radius PPG™, which offers Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, and Radius T°™, which provides continuous temperature measurements. Radius PCG requires no routine calibration, with accurate end-tidal carbon dioxide (EtCO2) and respiration rate measurements and continuous EtCO2 waveforms displayed within 15 seconds – all in a small, portable package that can fit in the palm of a hand.
"Radius PCG has been a game changer for our clinical team," commented Joseph DiMartino, MSN RN, NE-BC, CCRN-K, Associate Vice President of Nursing at Temple University Hospital in Philadelphia. "It provides us with a portable and rapid measure of capnography for confirming airway placement in accordance with AHA guidelines."
Wirelessly connected to Root, Radius PCG presents a compelling mainstream capnography solution, offering:
- Cable-free Capnography: High-quality capnography without a tethered connection to Root reduces the possibility of an interruption in capnography monitoring by minimizing tugging on the breathing circuit. In busy operating rooms, where space is already at a premium, and where capnography cables can easily be pulled and dropped on the floor—potentially damaging the fragile and expensive capnography sensor head—the reduction in clutter may be especially welcome.
- Automated Documentation: Root, in conjunction with the Masimo Hospital Automation™ Platform, automates electronic charting of patient data, including the data collected by Radius PCG, in hospital electronic medical record (EMR) systems, to simplify and speed workflows, as well as reduce the likelihood of transcription errors.1
- Maximized Data Visibility and Manipulation: Root's large, multi-touch, high-resolution screen provides an easily interpretable secondary display of large, crisp EtCO2 waveforms, improving visibility and assisting clinicians in identifying wave patterns suggestive of airway obstruction or tube dislodgement. Clearly displayed trend data for up to 96 hours helps clinicians review patient progress over time, helping guide ventilation efforts. And the intuitive touch-screen interface allows clinicians to quickly adjust the trend display range and configure alarm settings to meet the needs of each patient.
- Hassle-free Connectivity: Radius PCG quickly and effortlessly pairs with Root via Bluetooth, supporting seamless integration into clinical workflows while providing the benefits of reliable capnography.
Tom Friedland, MD, Emergency Medicine Physician, described Radius PCG as "the easiest and most affordable solution to switch your hospital from the unreliable color change CO2 detector to waveform capnography. #NoTraceWrongPlace."
"Radius PCG is indispensable for emergencies, as well as for monitoring the COVID patients in our house," added Kai Schurig, Head of the Biomedical Department at Marien Hospital in Hamburg, Germany. "These handheld devices are very reliable and fail very rarely. The users are very satisfied and treat the device accordingly."
Root is a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root’s plug-and-play expansion capabilities allow clinicians to simultaneously monitor with Radius PCG and many other measurements, such as Masimo SET®, advanced rainbow® Pulse CO-Oximetry measurements, O3® regional oximetry, and SedLine® brain function monitoring, for expanded visibility of patient status. Using Root in combination with the Hospital Automation Platform, monitoring data from all connected devices can be automatically charted in EMRs.
Joe Kiani, Founder and CEO of Masimo, said, "With its wireless connectivity, Radius PCG is a powerful and useful tool for assessing end-tidal CO2 in a multitude of clinical scenarios. Masimo continues to make clinically relevant, accurate patient data available, helping clinicians gain the insights they need to make the best decisions and improve patient outcomes."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- The Value of Medical Device Interoperability. West Health Institute. 2013.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Root® with Radius PCG™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Root with Radius PCG, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Study Investigates the Effects of Ventilatory Rescue Therapies on the Cerebral Oxygenation of COVID-19 Patients Using Masimo O3®
Neuchatel, Switzerland – April 6, 2021 - Masimo (NASDAQ: MASI) today announced the results of a prospective, observational study published in Critical Care in which researchers in Genoa, Italy evaluated the impact of a variety of rescue therapies on the systemic and cerebral oxygenation of mechanically ventilated COVID-19 patients suffering from acute respiratory distress syndrome (ARDS).1 To gauge the impact, the researchers used the Masimo Root® Patient Monitoring and Connectivity Platform with O3® Regional Oximetry, which uses near-infrared spectroscopy (NIRS) to enable monitoring of tissue oxygen saturation (rSO2) in the region of interest, such as the brain.
Dr. Chiara Robba and colleagues noted that “neurological complications are common in mechanically ventilated critically ill patients with COVID-19 and may lead to impaired cerebral hemodynamics,” and further, that respiratory rescue therapies “may have detrimental effects on brain physiology.” Observing, however, that there is currently little data available regarding the effect of rescue therapies on these patients’ brains, and in particular on cerebral oxygenation, the researchers sought to assess the impact of different ventilatory rescue therapies on the brain to help guide clinicians in choosing the most appropriate therapies for their COVID-19 patients.
The rescue therapies studied were recruitment maneuvers (RMs), prone positioning (PP), inhaled nitric oxide (iNO), and extracorporeal carbon dioxide removal (ECCO2R). To assess impact, the researchers measured (before and after the application of each method) arterial oxygen saturation (SpO2), partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), and cerebral oxygen saturation (rSO2). rSO2 was obtained using Masimo Root with O3, which also allowed them to observe several additional parameters unique to Masimo O3: ΔO2Hb, which monitors relative changes in the oxygenated hemoglobin component of rSO2; ΔHHb, which monitors relative changes in the deoxygenated hemoglobin component of rSO2; and ΔcHb, which monitors relative changes in total cerebral hemoglobin or blood volume. As a secondary aim, the researchers sought to evaluate the correlation between systemic and cerebral oxygenation.
The researchers found that the four rescue therapies had varied impact on cerebral oxygenation and the other measured parameters, noting in particular that after RMs, while there was no significant change in PaO2 or PaCO2, there was a significant decrease in rSO2. After PP and after iNO therapies, both PaO2 and rSO2 increased; ΔcHb also increased, corresponding to increased cerebral blood volume. After ECCO2R, both PaO2 and rSO2 decreased.
The researchers concluded, “Rescue therapies exert specific pathophysiological mechanisms, resulting in different effects on systemic and cerebral oxygenation in critically ill COVID-19 patients with ARDS. … The choice of rescue strategy to be adopted should take into account both lung and brain needs.”
They also noted, “To our knowledge, this is the first study investigating the early effects of rescue therapies on systemic and cerebral oxygenation and their correlation in critically ill patients with COVID-19-associated ARDS. The use of multimodal neuromonitoring, including new indices such as ΔHHbi + ΔO2Hbi, enabled us to better investigate the specific consequences of each ventilatory rescue strategy for brain and lung function. This is particularly important, especially in the early phases after rescue therapies application, when most of the effects on cerebral physiology are mainly acting.”
Dr. Robba and study co-author Dr. Basil Matta, Senior Medical Director at Masimo, commented, “The ability to observe relative changes in oxygenated, deoxygenated, and total hemoglobin with O3’s delta indices provided us with better insight into why brain saturations change as a result of interventions, and allowed us to better understand the interactions between systemic and cerebral hemodynamics. For example, we saw that turning patients prone resulted in improved systemic and cerebral oxygenation, whereas the lung recruitment maneuver did not improve systemic oxygenation, and even had an adverse effect by reducing brain oxygen saturation.”
They continued, “Above all, the main objective of improving the oxygen content of the blood is to deliver oxygen to vital organs, the most important of which is the brain. Masimo O3 provides the clinician with the ability to assess the impact of any medical intervention aimed at improving oxygenation. O3’s hemoglobin indices were critical to our understanding of the effects of our interventions on the brain. Without such a monitor, we are at best guessing, and in danger of flying blind. As we continue to seek to improve care and outcomes for patients with severe COVID-19, any tool that helps us better understand the impact of different medical interventions is most welcome.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Robba C, Ball L, Battaglini D, Cardim D, Moncalvo E, Brunetti I, Bassetti M, Giacobbe D, Vena A, Patroniti N, Rocco P, Matta B, Pelosi P. Early effects of ventilatory rescue therapies on systemic and cerebral oxygenation in mechanically ventilated COVID-19 patients with acute respiratory distress syndrome: a prospective observational study. Crit Care (2021)25:111. DOI: https://doi.org/10.1186/s13054-021-03537-1.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Root® with O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Root with O3, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Study Investigates the Impact of Automating Respiration Rate Measurement Using Masimo Rad-G™ with RRp®
Neuchatel, Switzerland - March 22, 2021 – Masimo (NASDAQ: MASI) today announced the results of a prospective, observational study published in Acta Paediatrica in which researchers from the Hospital for Sick Children in Toronto evaluated the accuracy of plethysmographic respiration rate measurement (RRp®) using Masimo Rad-G™, a rugged, handheld device, on malnourished, hospitalized children in Nigeria.1
Noting that in resource-limited environments, respiration rate (RR) measurement is often used to directly inform medical decisions for children with respiratory problems, but that manual RR counting "remains a challenge," Dr. Nancy Dale and colleagues investigated whether a technological solution might provide a useful alternative to manual counting. To make the evaluation, the researchers compared simultaneous device measurements and nurse-measured manual RR counts on malnourished children. The device chosen was the Masimo Rad-G, which uses a pulse oximetry sensor to measure both oxygen saturation and RRp, and which has been shown to provide good agreement between RRp and pediatrician-measured RR.2 They enrolled 514 children, aged 6 to 59 months, who were hospitalized between July 2019 and May 2020, in Borno State, Nigeria. Study nurses were trained to operate Rad-G and also perform manual RR counts as part of twice-daily patient assessment. RR was manually counted for 60 seconds while Rad-G simultaneously measured RRp via a sensor attached to the patient’s toe, and both measurements were recorded.
Analyzing the 6,889 paired RR measurements, the researchers found that the mean Rad-G RRp reading was 1.3 bpm (95% confidence interval 1.2 – 1.4 bpm) higher than the mean manual RR value. The mean absolute difference between the two methods was 4.4 bpm (95% CI 4.3 – 4.5 bpm). When RR was classified as either "normal" or "fast" breathing (using WHO pneumonia thresholds), the two methods resulted in the same classification 84% of the time. When RR was classified according to BedsidePEWS RR sub-scoring (a 4-point scale), 80% of the scores were the same, and 99.3% were within 1 point.
The researchers concluded that their findings "highlight the potential clinical impact of changing practice from manual to automated RR count. Clinical implementation of the device should be carefully monitored to measure impact on patient outcomes."
Study co-author Dr. Stanley Zlotkin commented, "Technical solutions to improve clinical care are laudable. We look forward to continuing this research."
RRp is one of multiple RR monitoring modalities offered by Masimo, which also include acoustic respiration rate (RRa®) and NomoLine® capnography (RRc™), helping clinicians ensure they have the most suitable tool for each patient scenario.
First developed in partnership with The Bill & Melinda Gates Foundation, Rad-G is a rugged, handheld device that provides clinically proven Masimo SET® pulse oximetry, respiration rate (RRp), and other important parameters. With its long-lasting rechargeable battery, robust rubber casing, and light weight, Rad-G makes it easier for clinicians to quickly assess patients and make informed care decisions anywhere pulse oximetry or vital signs checking is needed in a compact, portable form factor. Coupled with the universal Mini-Clip™ pulse oximeter sensor to provide the ultimate in handheld versatility, Rad-G can be used in a variety of settings, including limited-resource environments, both indoors and in the field.
In the U.S., RRp is 510(k) cleared for patients greater than two years old.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Dale N, Parshuram C, Tomlinson G, Shepherd S, Ashir GM, Maryah LB, Zlotkin S. Performance of automated versus nurse-measured respiratory rate measurements in hospitalized malnourished children. Acta Paediatr 2021. DOI: https://doi.org/10.1111/apa.15781.
- Alwadhi V, Sarin E, Kuma P et al. Measuring accuracy of plethysmography based respiratory rate measurement using pulse oximeter at a tertiary hospital in India. Pneumonia 2020;12:4. DOI: https://doi.org/10.1186/s41479-020-00067-2.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of RRp®, Rad-G™, and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including RRp, Rad-G, and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Sri Lankan Study Expands Evidence Demonstrating the Benefits of Critical Congenital Heart Disease (CCHD) Screening Using Masimo SET® Pulse Oximetry
Neuchatel, Switzerland - March 22, 2021 – Masimo (NASDAQ: MASI) today announced the results of a prospective study published in the Sri Lanka Journal of Child Health in which researchers in Colombo, Sri Lanka evaluated the efficacy of a pulse oximetry-based critical congenital heart disease (CCHD) newborn screening strategy using Masimo SET® pulse oximetry.1 The authors concluded that pulse oximetry is a "simple, noninvasive, cost-effective, feasible, and reliable test," and found that it had higher CCHD screening sensitivity than physical exam. Combining the two methods led to detection of all cases of CCHD in the study cohort, and they recommended that, "Pulse oximetry screening as a combined strategy with newborn physical exam should be implemented as a basic routine at discharge for every newborn in maternity units island-wide." As they note, their work is the first published CCHD study of this nature in Sri Lanka.
Noting that while "developed countries have abundant research on pulse oximetry screening" for CCHD, "there are few studies in developing countries," Dr. CR Gunaratne and colleagues sought to study the utility of such a screening strategy in their local setting. From November 2018 to April 2019, researchers assessed the rate of detection of CCHD using Masimo SET® pulse oximetry compared to routine physical exam alone in 5,435 asymptomatic newborns admitted to the post-natal wards at Castle Street Hospital, Colombo. Physical exam was performed at ≥ 24 hours of age "to identify any visible central cyanosis, weak/absent femoral pulses or cardiac murmur" by an experienced medical officer, blinded to pulse oximetry results. Radical-7® Pulse CO-Oximeters® with Masimo SET® pulse oximetry were used to measure pre-ductal and post-ductal oxygen saturation (SpO2) on the right hand and right foot, respectively, as part of a standardized screening algorithm. For newborns with positive results, an echocardiogram was performed within 48 hours to diagnose CCHD.
The researchers found that Masimo SET® pulse oximetry had a CCHD detection rate of 91%, compared to 82% for physical exam. The addition of Masimo SET® pulse oximetry to physical exam screening led to the detection of 2 cases missed by physical exam alone, with a combined detection rate of 100%. The positive predictive value and positive likelihood ratio were both higher for SET® pulse oximetry compared to physical exam (71.4% vs. 8.6% and 1232.7 vs. 46.2, p = 0.0001). The researchers also found that the false positive rate was "substantially" lower for SET® pulse oximetry compared to physical exam (0.07% vs. 1.76%, p = 0.0001).
The researchers concluded, "Prevalence of CCHD in our study was 2.02 per 1000 live births. Using a pulse oximetry strategy as an adjunct to routine physical exam can substantially reduce the diagnostic gap in CCHD as [a] combined approach has an additive effect resulting in more efficient screening."
Since its introduction in 1995, Masimo Measure-through Motion and Low Perfusion™ Signal Extraction Technology® (SET®) has been shown in more than 100 independent and objective studies to outperform other pulse oximetry technologies, providing clinicians with increased sensitivity and specificity to help them make critical patient care decisions.2 To date, nine other published CCHD screening studies, all with positive conclusions and representing over 300,000 infants, have used Masimo SET®,3-11 which includes the largest CCHD study to date, of 122,738 newborns.5 All of the CCHD studies with Masimo SET® pulse oximetry have shown improved screening sensitivity with the use of Masimo SET® alongside clinical assessment when compared to routine physical exam alone. Results from CCHD studies using other pulse oximetry technologies have shown that other technologies do not offer the same performance as Masimo SET® during CCHD screening.12-14
With its ability to accurately measure through motion and low perfusion, alongside its performance in outcome studies, SET® stands out as the choice of pulse oximetry technology for clinicians and policymakers hoping to implement newborn-related screening processes – and has indeed been used in the establishment of screening guidelines used around the world.15
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,16 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.17-20 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,21 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.22 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Gunaratne CR, Hewage I, Fonseka A, Thennakoon S. Comparison of pulse oximetry screening versus routine clinical examination in detecting critical congenital heart disease in newborns. Sri Lanka J Child Health, 2021; 50(1): 04-11. DOI: http://dx.doi.org/10.4038/sljch.v50i1.9393.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Slitine N, et al. Pulse Oximetry and Congenital Heart Disease Screening: Results of the First Pilot Study in Morocco. Int J Neonatal Screen 6(53). 30 June 2020.
- Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
- Ewer AK et al. Pulse Oximetry Screening for Congenital Heart Defects in Newborn Infants (Pulseox): A Test Accuracy Study. Lancet. 2011 Aug 27;378(9793):785-94.
- de-Wahl Granelli A et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr 2007; 96(10): 1455-9.
- Meberg A et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J Pediatr 2008; 152:761-5.
- Schena F et al. Perfusion Index and Pulse Oximetry Screening for Congenital Heart Defects. J Pediatr. 2017 Apr;183:74-79.
- Hamilçıkan S, Can E. Critical Congenital Heart Disease Screening With a Pulse Oximetry in Neonates. J Perinat Med. 2018 Feb 23;46(2):203-207.
- Jawin V et al. Beyond Critical Congenital Heart Disease: Newborn Screening Using Pulse Oximetry for Neonatal Sepsis and Respiratory Diseases in a Middle-Income Country. PLoS One. 2015; 10(9): e0137580.
- Tekleab AM, Sewnet YC. Role of pulse oximetry in detecting critical congenital heart disease among newborns delivered at a high altitude setting in Ethiopia. Pediatric Health Med Ther. 2019;10:83-88. https://doi.org/10.2147/PHMT.S217987.
- Narayen IC et al. Accuracy of Pulse Oximetry Screening for Critical Congenital Heart Defects After Home Birth and Early Postnatal Discharge. J Pediatr. 2018;197:29-35.
- Oakley JL et al. Effectiveness of Pulse-Oximetry in Addition to Routine Neonatal Examination in Detection of Congenital Heart Disease in Asymptomatic Newborns. J Matern Fetal Neonatal Med. 2015;28(14):1736-9.
- Kemper et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011 Nov;128(5):e1259-67. doi: 10.1542/peds.2011-1317.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
New Study Evaluates the Ability of Masimo EMMA® Capnography to Assess the Respiratory Status of Children with Tracheostomy
Neuchatel, Switzerland - March 15, 2021 – Masimo (NASDAQ: MASI) today announced the findings of an observational, retrospective study published in Pediatrics International. In the study, researchers at the Osaka Women’s and Children’s Hospital in Japan found the Masimo EMMA® Portable Capnograph "useful for assessment of the respiratory condition in children with tracheostomy."1 EMMA provides seamless mainstream capnography for patients of all ages in a compact, easily portable device. The device requires no routine calibration and minimal warm-up time, with accurate end-tidal carbon dioxide (EtCO2) and respiration rate measurements and continuous EtCO2 waveforms displayed within 15 seconds.
Noting the potential value of a compact and portable way to monitor changes in respiratory status for patients in scenarios where typical inpatient hospital monitoring equipment is less likely to be available, Dr. Masashi Hotta and colleagues sought to evaluate the utility of the EMMA capnograph on children with tracheostomy by comparing EtCO2 values from the EMMA device (which was connected to the distal side of the tracheostomy cannula) to invasively measured partial pressure of venous carbon dioxide (PvCO2). Although partial pressure of arterial carbon dioxide (PaCO2) is considered a gold standard for assessing respiratory condition, the researchers chose PvCO2 because "collection of arterial samples is more invasive than collection of venous samples" and noted that studies have shown a correlation between PaCO2 and PvCO2.2,3 They enrolled 9 infants (median age 8 months) and compared 43 paired EtCO2–PvCO2 readings in total.
The researchers found a correlation coefficient of 0.87 (95% confidence interval of 0.7 – 0.93; p < 0.001) between EtCO2 and PvCO2 readings. Analysis of the data revealed that EtCO2 readings were, on average, 10.0 mmHg lower than the corresponding paired PvCO2 value (95% limits of agreement of 1.0 – 19.1 mmHg). The researchers speculated that the tendency for EtCO2 to be lower than PvCO2 may be explained by "gas mixing proximal to the tracheostomy cannula due to the presence of anatomic and physiologic dead space. Because almost all patients used a cannula without a cuff, some air leakage may have occurred. In addition, about two-thirds of the patients had [chronic lung disease or bronchopulmonary dysplasia]," which they noted have been shown to cause lower CO2 concentrations during exhalation, relative to the partial pressure of CO2 in the blood.
They also found that the median difference in values was significantly greater for readings collected while patients were on mechanical ventilation (28 of the 43 data pairs). With a ventilator, there was a median 11.2 mmHg (6.8 – 14.3) difference; without a ventilator, there was a median 6.6 mmHg (4.1 – 9.0) difference (p = 0.043). The researchers noted that use of a ventilator was significantly related to the difference in paired readings because patients on ventilators had respiratory or circulatory disease.
Noting that "We demonstrated a strong positive relationship between PvCO2 and EtCO2 and revealed the availability and usefulness of this capnometer for children with tracheostomy," the researchers concluded, "EMMA is useful for assessment of the respiratory condition in children with tracheostomy. EMMA can be used especially in home-care settings and outpatient departments for such children." They also noted, "The main strength of this study is that we used a portable capnometer to evaluate EtCO2."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Hotta M, Hirata K, Nozaki M, Mochizuki N, Hirano S, and Wada K. Availability of portable capnometers in children with tracheostomy. Pediatrics Int’l. 2021. DOI:10.1111/PED.14516
- Fujimoto S, Suzuki M, Sakamoto K, et al. Comparison of End-Tidal, Arterial, Venous, and Transcutaneous PCO2. Respir Care. 2019;64(10):1208-14.
- Bloom BM, Grundlingh J, Bestwick JP, Harris T. The role of venous blood gas in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2014;21(2):81-8.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of EMMA®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including EMMA, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Masimo Monitoring Solutions Promote Newborn and Maternal Safety
New Neonatal Study Adds to Body of Clinical Evidence Demonstrating Masimo SET® Pulse Oximetry's Unique Ability to Improve Care
Neuchatel, Switzerland - March 8, 2021 – Masimo (NASDAQ: MASI) provides a variety of innovative monitoring solutions designed to improve maternal and newborn safety during childbirth and the critical first minutes of life. Masimo SET® pulse oximetry's ability to measure during motion and low perfusion has helped newborns, neonates, and pediatric patients like no other pulse oximetry. Not only has Masimo SET® helped clinicians reduce retinopathy of prematurity (ROP)1 and improve screening for critical congenital heart disease (CCHD),2-10 but it has helped push the standard of care for babies to new heights – the evidence from CCHD studies with SET®, for example, has been used in the establishment of screening guidelines used around the world.11
Today, on International Women's Day, it is especially important to recognize that, as UNICEF reports, "newborns and mothers are still dying in appalling numbers." Every day, approximately 7,000 babies in the first month of life die, and approximately 810 women die, from preventable complications related to childbirth or pregnancy.12 Similarly, according to the WHO, "Although important progress has been made in the last two decades, about 295,000 women died during and following pregnancy and childbirth in 2017. The most common direct causes of maternal injury and death are excessive blood loss, infection, high blood pressure, unsafe abortion, and obstructed labor, as well as indirect causes such as anemia, malaria, and heart disease."13
Masimo's first and ongoing focus has been helping neonatologists, pediatricians, OB-GYNs, and midwives around the world provide the best care possible for newborns and their mothers. The Masimo Newborn Sensor, the first and still the only sensor of its kind, was introduced in 2004 and is designed to provide accurate arterial oxygen saturation (SpO2) and pulse rate (PR) measurements in the fastest time possible during hectic neonatal resuscitation scenarios. Alongside Newborn Sensors, Pathway™, introduced in 2019 for the Root® platform, helps clinicians visualize their preferred SpO2 and PR protocol during neonatal resuscitation. Eve™, a software application introduced in 2014, simplifies and automates the CCHD screening process, which Masimo SET® enabled. The Blue® Sensor, introduced in 2004, provides accurate monitoring in cyanotic children at low SpO2 levels to help clinicians care for them and was validated specifically on infants with cyanotic disease.14 rainbow® SpHb®, noninvasive hemoglobin monitoring, introduced in 2008, can measure hemoglobin levels during pregnancy and alert clinicians to the possibility of excessive blood loss during delivery.
Adding to the significant body of clinical evidence demonstrating the utility of SET® pulse oximetry and other Masimo newborn and maternal solutions, a new study published in the Journal of Clinical Neonatology investigated the use of comparing Masimo perfusion index (Pi) pre- and post-ductal values on pre-term infants to aid clinicians in diagnosing hemodynamically significant patent ductus arteriosus (hsPDA).15 Dr. Melek Büyükeren and colleagues at Hacettepe University in Ankara, Turkey found that the difference in right-hand and right-leg Pi values obtained using Masimo SET® pulse oximetry was significantly higher in pre-term infants with hsPDA, leading them to conclude that the difference in Pi "has diagnostic value in hsPDA and can assist diagnosis when echocardiography is not available."
Marcelo Cardetti, MD, said, "As Head of the Neonatology Service of the Clinic and Maternity of the Center for Endocrinology and Human Reproduction (CERHU) in San Luis, Argentina, we have been using Masimo pulse oximetry monitors with SET® for approximately 8 years in all high-risk newborns and also for newborn resuscitation. In addition, we use Masimo SET® monitors for the detection of CHD and hypoxemia in all newborns in the mother-infant unit. Furthermore, our neonatal department is engaged in a research protocol on regional cerebral oxygenation (O3®) with neonatal sensors for Masimo Root- to know what happens with cerebral oxygenation during routine clinical procedures in the NICU. This monitoring, in addition to SpO2 and perfusion index (Pi), perfectly shows us what is happening with oxygenation of seriously ill newborns in real-time and in a noninvasive way. Masimo SET®'s innovative technology far overcomes the limitations of conventional oximetry and the Pi is an important clinical tool in the care of sick neonates. This monitor and the special neonatal RD sensors have been of great value for the prevention of ROP and for successful and quick, accurate, and reliable steps needed in resuscitation in the delivery room."
Hernando Baquero, MD, commented, "I am a pediatrician, neonatologist, clinician, educator and researcher in a major university in Colombia, with several publications on noninvasive neonatal SpO2 monitoring and oxygenation. The introduction in the Latin American market of Masimo SET® technology dramatically improved neonatal care in our countries. In contexts with serious resource limitations, as is the case in most neonatal units in our countries, it was vital to be able to provide quality care to the most vulnerable population due to their health conditions (e.g. hypoperfusion) or their biological characteristics (e.g. prematurity). Having reliable, fast, and stable readings as provided by SET® and its neonatal sensors improved the chances of many of our newborns."
Anne de-Wahl Granelli, PhD, Biomedical Scientist, RDCS(PE), Medical Centre Manager, Sweden, said, "The integration of pulse oximetry into the CCHD screening process has made a significant impact on the detection of congenital heart disease and neonatal health. In clinical studies, the use of pulse oximetry screening with SET® technology significantly improved the detection of duct dependent heart disease before hospital discharge. In 2011, the U.S. Department of Health and Human Services added pulse oximetry screening of newborns for CCHD to the Recommended Uniform Screening Panel. Today pulse oximetry has become a global standard of care when screening newborns for CHD."
Sergio Golombek, MD, MPH, FAAP, Member of the Board and Past President of the Ibero-American Society of Neonatology (SIBEN), said, "I have authored and published several scientific studies in relation to newborn oxygenation and screening for CCHD. Masimo SET® and new innovations and sensor development like RD sensors for noninvasive monitoring represent excellent technology that we can trust, that work promptly and accurately when we need it the most, and are designed specifically with ill newborn babies in the NICU in mind. SET® technology allows us also to do the pulse oximetry test or CCHD screening on newborn babies in our units, knowing well that we can fully trust the results. The technology is very easy to use and understand, and makes us deliver better clinical care."
Katsuyuki Miyasaka, MD, PhD, Executive Advisor, Wayo Women's University Graduate School, Tokyo and Professor Emeritus, St. Luke's International University, Tokyo, said, "As a critical care pediatric anesthesiologist, reliable and accurate pulse oximetry is paramount to optimal patient outcomes. Some suggest pulse oximetry is the fifth vital sign. Clinicians can rely on the sensitivity and specificity provided by Masimo's measure-through-motion technology in the management of children in the PICU. The use of pulse oximetry can lead to fewer adverse events in the recovery room by capturing accurate readings even during movement such as shivering in critically ill or unstable patients."
Mark Ansermino, MBBCh, MMed, Director of the Center for International Child Health and Professor, Department of Anesthesiology, Pharmacology & Therapeutics at the University of British Columbia, Canada, said, "Anemia is a significant public health problem that especially affects the quality of life, health status, and survival of mothers and children around the world. Having access to continuous hemoglobin monitoring technology can help provide visibility to hemoglobin levels. The noninvasive nature of the SpHb solution makes it comfortable for the mother and child and makes monitoring during childbirth feasible even in low-resource settings."
Asrat Dibaba Tolossa, MD, MPH, is Chief of Party for the Global Affairs Canada ENRICH (Enhancing Nutrition Services to Improve Maternal and Child Health) Program, a multi-year, multi-country initiative designed to improve the health and nutrition of mothers, newborns, and children. As part of the program, ENRICH has been conducting a study in central Tanzania, where maternal and child care services are often overburdened, using the Masimo Rad-67® Pulse CO-Oximeter®, which provides spot-check SpHb measurements. Dr. Tolossa commented, "In our field experimentation with the Rad-67, we found out that the device can be used easily by lower-level health workers in the communities for screening and referral of patients to health facilities for further assessment and treatment. There was also a high acceptance rate by community members as the method is noninvasive."
Joe Kiani, Founder and CEO of Masimo, said, "From our inception, we have been committed to improving outcomes for the youngest and most fragile patients. Our foundational SET® pulse oximetry was designed with newborns in mind. With rainbow® Pulse CO-Oximetry, we have made the noninvasive monitoring of child and mother clinically more meaningful. While we stand behind the fact that we have the best pulse oximetry for all patients, especially the most fragile patients, we continue to seek new ways to help clinicians provide newborns and their mothers with the best care possible. On this International Women's Day, we thank the caregivers who have dedicated themselves to the health of newborns and their mothers, as well as women everywhere, for their achievements, their sacrifices, and for nurturing us all."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgement considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Noninvasive, continuous SpHb has CE clearance for all patients and in the U.S. has received FDA clearance for patients >3 kg but is not currently indicated for patients <3 kg. As part of its U.S. FDA 510(k) clearance, spot-check SpHb on Rad-67 is contraindicated for use on pregnant patients and not indicated for use on pediatric patients or patients with renal disease. Eve has not obtained FDA clearance and is not available in the United States.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within +/- ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.16 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.17-20 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,21 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.22 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Slitine N, et al. Pulse Oximetry and Congenital Heart Disease Screening: Results of the First Pilot Study in Morocco. Int J Neonatal Screen 6(53). 30 June 2020.
- Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Ewer AK et al. Pulse Oximetry Screening for Congenital Heart Defects in Newborn Infants (Pulseox): A Test Accuracy Study. Lancet. 2011 Aug 27;378(9793):785-94.
- de-Wahl Granelli A et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr 2007; 96(10): 1455-9.
- Meberg A et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J Pediatr 2008; 152:761-5.
- Schena F et al. Perfusion Index and Pulse Oximetry Screening for Congenital Heart Defects. J Pediatr. 2017 Apr;183:74-79.
- Hamilçıkan S, Can E. Critical Congenital Heart Disease Screening With a Pulse Oximetry in Neonates. J Perinat Med. 2018 Feb 23;46(2):203-207.
- Jawin V et al. Beyond Critical Congenital Heart Disease: Newborn Screening Using Pulse Oximetry for Neonatal Sepsis and Respiratory Diseases in a Middle-Income Country. PLoS One. 2015; 10(9): e0137580.
- Kemper et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011 Nov;128(5):e1259-67. doi: 10.1542/peds.2011-1317.
- https://www.unicef.org/health/maternal-and-newborn-health
- https://www.who.int/health-topics/maternal-health#tab=tab_1
- Harris B et al. Ped Crit Care Med. 2016 Apr;17(4):315-20.
- Büyükeren M, Yiğit S, Aykan HH, Karagöz T, Çelik HT, Yurdakök M. Comparison of perfusion index and echocardiographic parameters in preterm infants with hemodynamically significant patent ductus arteriosus. J Clin Neonatol 2021;10:11-8.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- Turkish Journal of Medical Sciences in which Dr. Ayten Saracoglu and colleagues at the Marmara University Pendik Training and Research Hospital in Istanbul evaluated the ability of ORi™ to guide oxygenation by measuring its impact on hyperoxemia-mediated morbidity during one-lung ventilation (OLV) conducted as part of thoracic surgery.1 They concluded that ORi-guided oxygen titration "may reduce hospital stay and increase patient safety." " href="http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview" target="_blank;">http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of SET®, Newborn Sensors, Pathway™, Eve™, Blue®, rainbow®, and SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SET®, Newborn Sensors, Pathway, Eve, Blue, rainbow®, and SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Masimo Announces CE Marking of the Rad-G™ with Temperature
Neuchatel, Switzerland - March 2, 2021 – Masimo (NASDAQ: MASI) today announced the CE marking of the Rad-G™ with Temperature, a rugged handheld device that provides clinically proven SET® pulse oximetry, respiration rate from the pleth (RRp®), and other important parameters alongside clinical-grade, non-contact infrared thermometry. With its long-lasting rechargeable battery, robust rubber casing, light weight, and integrated noninvasive, real-time forehead temperature measurement, Rad-G with Temperature makes it easier for clinicians to quickly assess patients and make informed care decisions anywhere pulse oximetry or vital signs checking is needed in a compact, portable form factor. Coupled with the universal Mini-Clip™ pulse oximeter sensor to provide the ultimate in handheld versatility, Rad-G with Temperature can be used in a variety of settings, including but not limited to entry screening, physicians' offices, outpatient services, long-term care facilities, wellness clinics, first-response scenarios, and limited-resource environments both indoors and in the field. Rad-G can provide both spot-check measurement and continuous monitoring.
The infrared thermometry offered by Rad-G with Temperature provides a host of benefits. Rad-G's thermometer is non-contact and does not require probe covers or other disposable accessories. Its integration into the Rad-G platform eliminates the need for clinicians to locate a separate clinical thermometer to take body temperature measurements and ensures that many people can be seamlessly and efficiently screened for temperature, with one-touch operation, alongside oxygen saturation, respiration rate, and more, in the same session, using a single device. Designed from the start to maximize portability and battery life, Rad-G's rechargeable battery provides an impressive 24 hours of continuous use between charges – allowing clinicians to work in transport, emergency, and other challenging scenarios with confidence that the device will continue to function hour after hour.
First developed in partnership with The Bill & Melinda Gates Foundation as a spot-check device for use in pneumonia screening, the Rad-G with Temperature expands on its predecessor's capabilities not only with the ability to measure temperature, but the addition of alarms, and thus the ability to provide both continuous monitoring and spot-check measurement – without sacrificing any portability, convenience, or ruggedness. Using the included power adapter, Rad-G can be easily converted from a handheld, spot-check device into a continuous monitoring device, in the absence of other multi-parameter monitors. As of 2010 – twenty years after use of pulse oximetry during surgery became routine in affluent countries – more than 77,000 operating theaters in low- and middle-income countries were still conducting surgery without pulse oximetry.1 Working with a myriad of non-profit organizations, Rad-G is being made available at an affordable price so that the five billion people who don't have access to reliable pulse oximetry can finally have it. When used for continuous monitoring, the high-resolution screen displays a continuous pleth waveform and the fully configurable, audible alarms help alert clinicians to changes in patient status that may require their intervention.
The development of Rad-G stems in part from the findings of a multi-center, prospective, two-stage observation study funded by The Bill & Melinda Gates Foundation, whose protocols were published in JMIR Research Protocols, in which Dr. Kevin Baker, MA, MS.c, Senior Research Specialist at the Malaria Consortium, and colleagues sought to identify the most accurate, usable, and acceptable devices to aid community health workers in the diagnosis of pneumonia symptoms in resource-poor settings.2 The researchers found that "The Masimo mobile phone pulse oximeter [iSpO2® Rx] had the best overall performance across all measures and in both age strata of the children the device was tested on. This may be due to the motion signal processing techniques incorporated in Masimo pulse oximeters which attempts to reduce motion artefact, which may be particularly important when using these devices on moving children."3
Paul Farmer, Kolokotrones University Professor at Harvard, Chair of the Department of Global Health and Social Medicine at Harvard Medical School, Chief of the Division of Global Health Equity at Brigham and Women's Hospital in Boston, and Co-Founder and Chief Strategist of Partners in Health, said, "In the places where I've worked around the world, there has always been a demand for tools that enable the continuous monitoring of key vital signs, like respiration rate, oxygen saturation, and temperature, which can help providers and patients fight against illnesses from pneumonia to congenital heart disease."
Eric D. McCollum, MD, MPH, Director of the Global Program in Respiratory Sciences at the Johns Hopkins School of Medicine in Baltimore, Maryland, said, "The Masimo Rad-G is a fantastic device that is thoughtfully crafted and user-friendly for both healthcare workers with diverse training backgrounds and pediatric patients across the age spectrum. We are using the Rad-G currently in four countries in our pediatric global health work and the device is no doubt at the high standards set by Masimo with its range of high-quality pulse oximeters. The healthcare providers and children love it."
"Bacterial and viral pneumonias – including those caused by COVID-19 – are a leading cause of death in children and adults globally, with a disproportionate burden of disease in low-resource settings," said Peter Moschovis, MD, MPH, a pulmonologist at Massachusetts General Hospital. "Pulse oximetry plays an important role in the triage and management of patients with pneumonia."
Joe Kiani, Founder and CEO of Masimo, said, "With Rad-G, we set out to create an accessible, high-quality care solution that clinicians can rely on in a multitude of care settings to serve the five billion people on our planet that to date have not had access to pulse oximetry, let alone SET® pulse oximetry. With the addition of temperature measurement, Rad-G is more versatile than ever, streamlining the assessment of multiple key vital signs. Many caregivers travel miles, sometimes on bike, sometimes on foot, to help patients, so having a product that is light, small, multifunctional, and 'accurate when you need it most' is crucial. Rad-G was designed to be just that."
SpO2 and PR monitoring on Rad-G is provided using clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other technologies.4 SET® is estimated to be used on more than 200 million patients a year5 and is the primary pulse oximetry at 9 of the 10 hospitals that top the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.6 With Masimo SET® technology in Rad-G, clinicians have access to accurate pulse oximetry measurements in the palm of the hand.
In a new cross-sectional study published in Acta Paediatrica, Dr. Baker and colleagues assessed the utility of Rad-G by observing how it was used by healthcare workers screening children under five for pneumonia in three regions of Ethiopia in 2018.7 The researchers found that healthcare workers gave correct treatment and referral guidance using Rad-G's results and their assessment of other symptoms in 94.9% and 95.8% of cases in the first and second of their two observation groups, respectively.
In addition to temperature measurements and Masimo SET® oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and PVi® (for assessing fluid responsiveness), the same SpO2 sensor can be used to monitor respiration rate from the plethysmograph, with RRp. Difficulty breathing and fever are generally considered two of the earliest signs of patient deterioration, and Masimo hopes that the availability of RRp and thermometry on Rad-G may play a role in assisting clinicians and public health officials as they seek to combat numerous types of illnesses, including pneumonia and COVID-19.
Rad-G with Temperature can be used with a variety of reusable and single-patient use sensors. The universal direct-connect Rad-G reusable sensor, indicated for monitoring adult, pediatric, and infant patients, helps to eliminate the need to stock and carry multiple sensor types, increasing the device’s versatility and ease of use, especially in more challenging field environments. Rad-G with Temperature is also compatible with the vast portfolio of Masimo single-patient-use adhesive sensors – including Masimo RD SET® sensors, which offer best-in-class accuracy specifications of 1.5% in conditions of motion and no motion – ensuring clinicians can customize their setup based on the unique needs of each care setting. In addition, Rad-G is designed to work reliably on all people, from white to black, neonate to geriatric.
Rad-G is FDA 510(k) cleared and is available in the U.S. Rad-G with Temperature has not received FDA 510(k) clearance and is not currently available in the U.S. PVi is FDA 510(k) cleared as an indicator of fluid responsiveness in select populations of mechanically ventilated adult patients in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.10-13 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,5 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.6 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- https://www.theatlantic.com/health/archive/2017/
02/pulse-oximeter/516510/ - Baker K, Akasiima M, Wharton-Smith A, Habte T, Matata L, Nanyumba N, Okwir M, Sebsibe A, Marasciulo M, Petzold M, Källander K. “Performance, Acceptability, and Usability of Respiratory Rate Timers and Pulse Oximeters When Used by Frontline Health Workers to Detect Symptoms of Pneumonia in Sub-Saharan Africa and Southeast Asia: Protocol for a Two-Phase Multisite, Mixed-Methods Trial.” JMIR Res Protoc. 2018;7(10):e10191) doi: 10.2196/10191.
- https://openarchive.ki.se/xmlui/bitstream/
handle/10616/46833/Thesis_Kevin_Baker.pdf?sequence=4&isAllowed=y - Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
- Baker K, Ward C, Maurel A, de Cola M, Smith H, Getachew D, Habte T, McWhorter C, LaBarre P, Karlstrom J, Ameha A, Tariku A, Black J, Bassat Q, Kallander K. “Usability and acceptability of a multimodal respiratory rate and pulse oximeter device in case management of children with symptoms of pneumonia: A cross-sectional study in Ethiopia.” Acta Paediatrica. 19 Nov 2020. DOI: 10.1111/apa.15682
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-G™, SET®, RRp®, and iSpO2® Rx. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-G, SET®, RRp, and iSpO2 Rx, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Masimo Announces U.S. Release of softFlow® High-Flow Nasal Cannula Therapy
Innovative Nasal High-Flow Therapy Provides Respiratory Support for Patients with COVID-19 and Other Respiratory Conditions
Irvine, California, California - February 23, 2021 – Masimo (NASDAQ: MASI) today announced the U.S. introduction of softFlow®, innovative pulmonary care therapy which provides nasal high-flow warmed and humidified respiratory gases to spontaneously breathing patients. The technology, available on the softFlow 50, offers adult patients high-flow respiratory support through a soft nasal cannula by generating a consistent high flow of warm, humidified air or air/oxygen mixture.
As the COVID-19 pandemic continues, increasing the number of patients suffering from respiratory conditions and requiring respiratory support, softFlow offers clinicians across the continuum of care an important tool to help in the treatment of spontaneously breathing patients. Capable of operating without an external compressed air supply, softFlow is designed for maximum versatility, with utility in settings throughout the hospital, long term-care facilities, and use at home. To reduce the risk of cross-contamination, softFlow uses a single-patient-use flow path from the internal mixing chamber to the patient and a bacterial/viral filter designed to filter contaminants from the air delivered to the patient.
The U.S. National Institutes of Health (NIH) and World Health Organization (WHO) have both suggested that the use of High-Flow Nasal Cannula (HFNC) therapy, like softFlow, is a viable option for providing respiratory support for select COVID-19 patients for whom conventional oxygen therapy may be insufficient.1 softFlow can provide patients suffering from COVID-19 or other respiratory conditions with higher flow rates of oxygen than conventional oxygen therapies. As a therapy, NIH concluded, "HFNC is preferred over Noninvasive Positive Pressure Ventilation (NIPPV) in patients with acute hypoxemic respiratory failure based on data from an unblinded clinical trial in patients without COVID-19 who had acute hypoxemic respiratory failure."2 The referenced study found a higher number of ventilator-free days (24 days) with HFNC than with conventional oxygen therapy (22 days) or NIPPV (19 days) (p=0.02). The researchers also found a lower 90-day mortality rate compared to conventional oxygen therapy or NIPPV.3
With softFlow, the airflow is consistently delivered through the nose, allowing patients to continue to eat, drink, and speak, which is difficult with mask-based forms of respiratory support. In addition, the softFlow 50 system provides warm, humidified gas into the patient's nose, to enhance comfort and aid in mucous clearance.4,5 With its ability to precisely deliver high respiratory gas flow rates well above those required for typical respiratory demand, clinicians can also take advantage of the high-flow rate to help limit the entrainment of room air (which can reduce the quantity of delivered inhaled oxygen).
The innovative integrated airflow generator of the softFlow technology and water reservoir attachment allow for continued HFNC treatment of recovering COVID-19 patients in the home and other care settings, without the need for a separate source for high-flow air like other devices. The device's simple, intuitive interface allows patients or their caregivers (who can lock settings) to easily customize the flow rate and humidification level.
First available in 2016, softFlow is now in use in numerous countries in Africa, Asia, and Europe.
Beijing Aerospace Changfeng Co., LTD, of China, noted, "We are very satisfied with the therapy successes we achieve with this device. We would like to emphasize the stable flow during inhalation and exhalation, which is generated by the powerful motor, which doesn’t need an external air source, as well as the resulting CO2 washing out. This fact opens up possibilities for [use as] a therapy for hypercapnic patients. Another very positive aspect of the device is the way it prevents condensation in the applicator by warming the entire tube system up to the nasal cannula. … All in all we are very satisfied with the handling of the device and the medical results."
Miguel Marina Barrio, Product Manager, Intensive Care Division, Hospital Hispania, Spain, added, "We already ordered 50 devices and we are very satisfied with the way softFlow performed. … In our opinion, the following points are very good: menu structure, product features, design, usability, manuals, delivery times. … The product quality, customer service, and type of packaging are particularly excellent."
Dr. Dalal Al Matrouk, Head of Anesthesia and ICU at Farwaniya Hospital, Ministry of Health, Kuwait, said, "We have deployed 10 units of Masimo softFlow to help our clinicians manage patients with COVID-19-induced respiratory problems. We realize that high-flow nasal therapy could potentially help avoid invasive mechanical ventilation and its associated risks of ventilator-induced lung injury and hospital-acquired pneumonia."
Joe Kiani, Founder and CEO of Masimo, said, "We believe softFlow provides clinicians with an important tool to help address the growing number of people with compromised respiratory function, whether in high-acuity or low-acuity settings, including at home. We're happy to now be able to offer this technology in the United States."
The softFlow 50 is FDA cleared for use in hospital and long-term care facilities. Home use is being made available in the US under the FDA Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the COVID-19 Public Health Emergency.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.6 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,7 improve CCHD screening in newborns,8 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.9-12 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,13 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.14 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf
- https://www.covid19treatmentguidelines.nih.gov/critical-care/oxygenation-and-ventilation/
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25981908.
- Hasani A et al. Chron Respir Dis 5, no. 2 (2008): 81-86.
- Roca O et al. Respir Care 55, no. 4 (2010): 408-413.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements: Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo softFlow®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo softFlow, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Masimo Announces Full Market Release of Masimo SafetyNet-OPEN™
Easy-to-Implement Solution Helps Businesses, Schools, and Other Organizations Reopen Responsibly and Stay Open Safely During Infectious Illnesses
Irvine, California, California - February 15, 2021 – Masimo (NASDAQ: MASI) today announced the full market release of Masimo SafetyNet-OPEN™, a web and mobile app solution that helps businesses, schools, and other organizations screen, trace, and manage users as they face COVID-19 and other infectious illnesses, such as seasonal flu. SafetyNet-OPEN not only helps organizations bring their people back to the workplace responsibly, but stay open safely. Tailored for each organization’s safety protocols and needs, SafetyNet-OPEN is capable of covering all stages of back-to-work management, including risk screening, exposure contact tracing, and recovery management.
As a global leader in noninvasive patient monitoring technologies and advanced connectivity and automation solutions, Masimo is uniquely positioned to provide organizations with the tools to assist them in staying open safely. Trusted by leading hospitals to monitor more than 200 million patients each year,1 Masimo, in the first phase of the COVID-19 pandemic, developed the Masimo SafetyNet™ remote patient management solution, now in use at hospitals around the world, to help keep patients and frontline workers safe. SafetyNet-OPEN builds on SafetyNet by scaling this patient management to the level of entire organizations, no matter the size. When escalation of care is needed, SafetyNet-OPEN can even integrate clinical monitoring using Masimo SafetyNet, in partnership with the organization's health care provider or local hospital.
Masimo SafetyNet-OPEN helps organizations identify those who are most at risk of COVID-19 or other infectious viruses, trace possible exposure to limit the spread of the virus, and monitor users' vital signs, including temperature, to help detect the onset of fever, and arterial oxygen saturation, for signs of dangerous deterioration. Based on answers to daily questions and physiological data from connected monitoring devices, users receive a personalized daily risk score, automating directives to stay home, get tested, or seek treatment, configured according to each organization’s safety protocols, back-to-work procedures, and changing local health authority guidelines.
Flexible and versatile, the Masimo SafetyNet-OPEN system can be augmented with additional components to offer more advanced management of the organization's users. To help screen potentially infected users, results from on-site testing, implemented in coordination with a trusted lab partner, can be securely stored and taken into account in risk scoring and assessment. To improve contact tracing, SafetyNet-OPEN can be customized with proximity wristbands or with the Radius T°™ wearable continuous thermometer for both temperature measurement and proximity analysis, to help track users' exposure to those who may be at risk, including time spent in close contact. And when care escalation may be needed, the organization, in collaboration with a trusted clinical partner, can easily incorporate vital signs data into SafetyNet-OPEN, to monitor impacted users as their risk level rises or during recovery from COVID-19, flu, or another illness. Data can be collected from the Radius T° continuous thermometer (to help track fever), the Radius PPG™ wearable continuous pulse oximeter, and other monitoring devices.
For businesses, schools, and organizations both large and small, Masimo SafetyNet-OPEN is easy and fast to implement, with no desktop software to install and with a dedicated Masimo support team to ensure seamless rollout. A web-based workforce management dashboard provides a clear picture of every team member's status – who's at risk, who's recovering, who's cleared to work, proximity violations, etc. – helping organizations efficiently and safely manage their teams during COVID-19 and beyond. Every aspect, from the daily risk-assessment questions, to the rules of the algorithm that determines risk scores, to group assignments for contact tracing, and more, can be easily customized. Furthermore, SafetyNet-OPEN is designed to ensure user data is secure.
Dr. Richard Carmona, MD, MPH, 17th Surgeon General of the United States and Distinguished Professor of Public Health at the University of Arizona Mel and Enid Zuckerman College of Public Health, said, "One of Masimo SafetyNet-OPEN's greatest strengths is its great versatility and customizability, which gives it enormous potential to provide utility in so many settings. As a former Surgeon General I can see tremendous application across a number of federal agencies whose day-to-day operation and 24/7 capability we all depend upon. At the University of Arizona, where I lead the effort to safely reopen our campuses, we've been involved in numerous initiatives examining how COVID-19 affects social groups – for example, the effects of asymptomatic carriers, and how to prevent 'superspreader' events. SafetyNet-OPEN's risk assessment and mitigation tools, which can be fine-tuned to accommodate our evolving understanding of the disease, are ideal for better understanding and coping with such a complex challenge."
Claremont McKenna College President Hiram E. Chodosh, JD, said, "We are grateful to Masimo for the pilot use of its SafetyNet-OPEN tools, which we believe provide yet another important way for us to strengthen the health and safety capabilities of our college campus and the broader community."
Dr. Mark Ferris, MSc, MRCGP, MFOM, Occupational Physician, UK, commented, "Over the last year, we have gained considerable knowledge about COVID-19 and how best to control it. It is clear that to protect our colleagues or students, their families, our workplaces, and the wider community, we need to combine as many strategies as possible. These include the provision of accurate information, physical distancing, masks, ventilation, hygiene precautions, testing and monitoring, contact tracing, and supported isolation. The Masimo SafetyNet-OPEN solution offers a vital way to help organizations apply this combined strategy."
Filmmaker Mark Kassen, Co-Founder and Executive Chairman of Like Minded Media Ventures, added, "We are all anxious to get back to work but without Masimo SafetyNet-OPEN, which we used during its limited market release, we never would have felt safe and secure enough to ask our cast and crew to come back to set."
Dr. Oscar San Román Orozco, MD, COVID-19 Clinic, University Health System, Universidad Autonoma de Queretaro, Mexico, explained, "Public Health interventions never work in silos, because pandemics don’t just affect one sector. To overcome the challenges that COVID-19 has given us, we need a robust systematic approach that involves intersectoral, evidence-based interventions and technology. Masimo SafetyNet-OPEN provides employers and university leaders with the necessary tools to make their communities feel safe through an organized, controlled, and monitored strategy. We might not eradicate the disease soon, but we can control it by segmenting work and social groups based on their risk, monitoring these social bubbles using a robust screening and contact tracing method, and trusting in new intersectoral approaches and technologies. These will allow us to delimit the viral propagation and maintain tight control in case any individual gets infected."
Joe Kiani, Founder and CEO of Masimo, said, "As the COVID-19 pandemic continues, organizations around the world are struggling with the immense challenge of balancing the health and safety of employees, students, and members with the economic and educational needs of their communities. Masimo has developed numerous technologies to help patients and clinicians stay safe during the pandemic. With SafetyNet-OPEN, we've combined our decades of expertise in patient monitoring with our latest innovations in automation and connectivity to help organizations confront this challenge and better manage future ones."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,1 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements: Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet-OPEN™, Radius T°™, and Radius PPG™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet-OPEN, Radius T°, and Radius PPG, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Masimo Expands Suite of Advanced Measurements Through Acquisition of UK-Based LiDCO Group Plc
Leading Provider of Hemodynamic Monitoring Becomes Masimo Subsidiary
Irvine, California, California - February 2, 2021 – Masimo (NASDAQ: MASI) today announced that it has successfully completed the acquisition of the LiDCO Group Plc, a leading provider of advanced hemodynamic monitoring solutions (LiDCO). The Company completed the acquisition of 100% of LiDCO on February 2, 2021.
LiDCO was founded in 1991, launched its first product in 1999, and began trading on the AIM submarket of the London Stock Exchange in 2001. On January 11, 2021, LiDCO was de-listed from AIM and will be re-registered by Masimo as a UK-based private subsidiary.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates3 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About LiDCO
LiDCO is a supplier of noninvasive and minimally invasive hemodynamic equipment used to monitor the amount of blood flowing around the body and ensure that vital organs are adequately oxygenated. Clinical studies show that optimizing the hemodynamic status of high-risk patients produces better outcomes and reduced hospital stay. Since launching our first product in 1999, LiDCO has been committed to improving patient outcomes whilst providing excellent customer service. Dedicated to making a difference.
Forward-Looking Statements: Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, those related to Masimo’s proposed acquisition of the Connected Care Business from NantHealth (the "Acquisition"), and are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to achieving the conditions to closing the Acquisition and risks regarding the integration of assets acquired from NantHealth; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces the iSirona™ Connectivity Hub
Irvine, California – January 19, 2021 – Masimo (NASDAQ: MASI) today announced the global launch of iSirona™, a compact, versatile connectivity hub designed to maximize interoperability across the continuum of care. The iSirona hub offers an efficient way to physically connect up to six medical devices at the bedside and automatically route the data to the Masimo Hospital Automation™ platform, a vendor-agnostic connectivity solution for EMR integration, surveillance monitoring, alarm management, mobile notifications, smart displays, and analytics. Supplemented by iSirona, Masimo Root® – a powerful, versatile, multimodal patient monitoring and connectivity solution combining numerous advanced measurements with sophisticated expansion capabilities – helps ensure that whatever the source, all patient data can be accurately and efficiently captured and presented to clinicians in the most suitable ways.
iSirona, Root, and the Hospital Automation platform are already compatible with an ever growing list of more than 500 medical devices. With its focus on versatility and compatibility, iSirona is particularly valuable as an easy but robust way for hospitals to connect almost all medical devices, regardless of brand – helping reduce the time spent manually charting patient data and instances of transcription errors while promoting efficient workflows by giving clinicians access to patient data wherever, whenever, and however they want to see it.
With its compact and fan-less design, iSirona is ideal for connecting multiple patient monitors, anesthesia machines, pumps, and other medical devices in operating rooms and ICUs where space is often restricted. The included multi-slotted mounting bracket allows iSirona to be fixed in a room or mounted directly to a mobile medical device (such as a ventilator) to provide connectivity wherever the medical device is used. The onboard rechargeable battery and local storage allow the iSirona to continue operating and buffering data for up to two hours in the event of a power outage or loss of network connectivity, helping reduce potential loss of data during emergencies and ensuring data continuity during patient transport. With software-configurable USB and Bluetooth® connections, iSirona is designed to integrate today's physical medical devices and support wireless and wearable devices in the future, as well as provide utility in both high- and low-acuity areas throughout the hospital.
In addition to iSirona and Root, the Hospital Automation platform encompasses a variety of components that together provide a holistic, end-to-end hospital data automation solution that aggregates high-fidelity parametric data, waveforms, and alarms from Masimo and third-party devices. For example, clinicians can surveil and remotely monitor patients from a central location, as well as view alarms, using Masimo Patient SafetyNet™. With Masimo Replica™, real-time monitoring data and alarm notifications can be relayed to mobile devices, reaching clinicians wherever they are – even beyond the hospital. Masimo UniView™ brings together data from multiple devices on an enlarged display that can be tailored for each patient case, helping ensure that data is visualized for clinical teams as usefully as possible. And Masimo Iris Analytics™ transforms data into richly detailed, customizable patient and even hospital-wide reports.
Joe Kiani, Founder and CEO of Masimo, said, "We believe that Masimo's Hospital Automation platform is the most customizable, versatile, and future-focused connectivity solution on the market. We continue to deepen our understanding of what data clinicians most want to have at their fingertips and how, in the ideal hospital of tomorrow, it will be available to them – and to develop monitoring, display, automation, notification, and reporting solutions that best address these needs now. iSirona, the latest member of the Hospital Automation family, makes it easier than ever to add connectivity wherever it's needed – bringing offline data online, smoothing workflows, and ultimately, helping clinicians improve patient care."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo iSirona™, Hospital Automation™, and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo iSirona, Hospital Automation, and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]