News & Media-2019
2019
Home / News & Media / 2019
2019
NEWS & MEDIA:

Masimo Announces FDA Clearance for Neonatal RD SET® Pulse Oximetry Sensors with Improved Accuracy Specifications
Improved SpO2 Accuracy Now Available for All Patient Populations
Irvine, California - December 30, 2019 – Masimo (NASDAQ: MASI) announced today that RD SET® sensors with Masimo Measure-through Motion and Low Perfusion™ SET® pulse oximetry have received FDA clearance for improved oxygen saturation (SpO2) accuracy specifications for neonatal patients (< 3 kg). The updated RD SET® sensors' SpO2 accuracy specifications have improved significantly, from 3% to 1.5% ARMS1 (at 1 standard deviation), in conditions of motion and no motion, providing clinicians with even greater confidence when monitoring the oxygenation status of neonates. With this clearance, the improved performance specifications, which were incorporated into RD SET® sensors for patients > 3 kg in 2018, are now available to all patient populations in the United States.
-
 Masimo RD SET® Sensors for Neonates
Masimo RD SET® Sensors for Neonates
Masimo's revolutionary SET® pulse oximetry has been shown in more than 100 independent and objective studies to outperform other pulse oximetry technologies – even before the revisions that achieved the improved accuracy specifications – providing clinicians with increased sensitivity and specificity to help them make critical patient care decisions.2 Crucially for newborn health, SET® has been shown to help clinicians reduce severe retinopathy of prematurity in neonates3 and in multiple studies, including the largest critical congenital heart disease (CCHD) study to date, to improve CCHD screening in newborns.4-5
In addition to offering improved accuracy, RD SET® sensors are designed to enhance patient comfort, optimize clinician workflows, and help hospitals meet green initiatives. The sensors are lightweight and have a flat, soft cable with smooth edges, so that they lie comfortably on a patient's hand or foot. In particular, RD SET® NeoPt sensors with Velaid SofTouch™ use little to no adhesive, facilitating quick but gentle application and repositioning on the fragile skin of newborns and pre-term babies. RD SET® sensors also feature an intuitive sensor-to-cable connection, while their lightweight design results in up to 84% less waste and their sleek, recyclable packaging reduces storage and shipping space.
Joe Kiani, Founder and CEO of Masimo, said, "We're delighted to announce the latest result of our continued innovation in our foundational SET® pulse oximetry. We have long been dedicated to helping improve the lives of neonatal, infant, and pediatric patients, and this clearance significantly furthers that mission. Thanks to the brilliance and dedication of our engineers and the continuing support of our customers, we’ve been able to once again raise the standard for pulse oximetry performance. Even though no one has been able to create pulse oximetry that outperforms SET®, we have not allowed that to stop us from continuing our pursuit of perfecting pulse oximetry.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-8 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
6. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
7. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
8. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
9. Estimate: Masimo data on file.
10. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® and RD SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo SET® and RD SET® contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo and Dräger Expand Licensing Agreement to Add Masimo Brain Monitoring and Capnography Technologies
Irvine, California and Lübeck, Germany - December 19, 2019 – Masimo (NASDAQ: MASI) and Dräger (OTCMKTS: DRWKF) announced today that they have expanded their partnership, whereby Dräger will integrate additional Masimo measurement technologies into Dräger’s family of multi-parameter patient monitors, to help clinicians assess brain function, oxygenation, and ventilation status.
After becoming the first patient monitoring company to integrate Masimo noninvasive, continuous rainbow SET® technologies, including total hemoglobin (SpHb®), in its patient monitors, Dräger will add Masimo SedLine® Brain Function Monitoring, O3® Regional Oximetry, and NomoLine® Capnography measurements to its already comprehensive suite of advanced measurement parameters, tailored for operating rooms and critical care settings.
The three additional technologies are currently available directly from Masimo on the Root® Patient Monitoring and Connectivity Platform:
- • Next Generation SedLine Brain Function Monitoring, which assists clinicians in monitoring the state of the brain under anesthesia, with bilateral data acquisition and processing of EEG signals and an enhanced Patient State Index (PSi).
- • O3 Regional Oximetry, which may help clinicians monitor cerebral oxygenation in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain.
- • NomoLine "no-moisture" sampling lines, which are designed for low-flow applications and can be used in a variety of clinical scenarios and care settings, on both intubated and non-intubated patients of all ages, in both low- and high-humidity applications.
Stefan Dräger, CEO of Dräger, said, "For over 20 years, the foundation of our strong relationship with Masimo has been our shared vision to improve patient outcomes while lowering the cost of healthcare. This expanded agreement allows us to offer our customers the most innovative technologies, helping them to deliver even better patient care and achieve better patient outcomes."
Joe Kiani, Founder and CEO of Masimo, said, "Masimo's technical prowess in monitoring – from pulse oximetry, to noninvasive hemoglobin, to brain monitoring, and more – makes our technologies an ideal solution not just for our own devices but for other companies wanting to provide hospitals and patients the best possible care. Dräger has long understood this and we appreciate our longstanding partnership and their continued faith in us."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3.de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Estimate: Masimo data on file.
8. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Dräger. Technology for Life.®
Dräger is an international leader in the fields of medical and safety technology. Our products protect, support and save lives. Founded in 1889, Dräger generated revenues of around EUR 2.6 billion in 2018. The Dräger Group is currently present in more than 190 countries and has more than 14,000 employees worldwide. Please visit www.draeger.com for more information.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®, O3®, NomoLine®, and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo SpHb and Radical-7, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Dräger
Melanie Kamann
Phone: +49 451 882-3998
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Multicenter Study Evaluates Trend Accuracy of Noninvasive, Continuous Masimo SpHb® and Two Invasive Hemoglobin Testing Methods
Irvine, California - December 9, 2019 – Masimo (NASDAQ: MASI) announced today that in a multicenter study recently published in the Journal of Clinical Monitoring and Computing, researchers at three institutions – Loma Linda University in California (LLU), the University of California at Irvine (UCI), and Mayo Clinic in Jacksonville, Florida (MCF) – evaluated the trend accuracy of three hemoglobin (Hb) monitoring methods, including noninvasive, continuous Masimo SpHb®.1
-
 Masimo Radical-7® with SpHb®
Masimo Radical-7® with SpHb®
Dr. Applegate and colleagues prefaced their investigation by noting that Hb measurement "informs patient-specific perioperative transfusion decisions within the context of symptoms, comorbid conditions, surgical procedure, observed bleeding and hemodynamic performance." They also noted, however, that "the time needed for blood sampling and analysis can cause Hb measurement to lag clinical situations. In surgical settings in which blood loss may not be apparent or be difficult to estimate, continuous rather than intermittent Hb monitoring could provide earlier warning of decreasing Hb." Thus, they sought to determine whether noninvasive, continuous hemoglobin monitoring using Pulse CO-Oximetry (SpHb) might provide useful real-time information about changes in Hb.
The researchers compared noninvasive SpHb measurement and two invasive methods of determining intraoperative Hb changes – arterial blood gas CO-oximetry (ABGHb) and point-of-care hemoglobin using arterial blood (aHQHb) – to laboratory determined hemoglobin changes (tHb) for trend accuracy. SpHb was measured using Masimo Radical-7® Pulse CO-Oximeters® with rainbow® fingertip sensors at all sites. Based on the institution, ABGHb was measured using either a Radiometer ABL800, Nova Biomedical CCX or PhOX, or Siemens RAPIDLab 1265; aHQHb was measured with a HemoCue HB 301; and tHb was measured using either a Sysmex XE5000 or Coulter AcT-diff or LH 750, also depending on the institution.
The researchers independently enrolled 135 adult patients undergoing non-cardiac surgery in which arterial catheterization was planned and repeated intraoperative blood gas analysis was expected (51 at LLU, 26 at UCI, and 58 at MFC). During surgery, whenever arterial blood analysis was performed, SpHb (as displayed at the time blood was drawn) was recorded, and samples were analyzed within ten minutes using ABGHb, aHQHb, and tHb. On average, patients had 4 samples obtained (ranging from 2 to 13), with a total of 551 blood gas samples analyzed, providing 416 sequential changes in Hb for trend assessment.
Using modified Bland-Altman analysis, the researchers assessed trend accuracy for the three methods compared to laboratory analysis, calculating mean bias (95% limits of agreement) of 0.10 (-1.14 to 1.35) for SpHb, -0.02 (-1.06 to 1.02) for ABGHb, and 0.003 (-0.95 to 0.95) for aHQHb. Defining a change in SpHb, ABGHb, or aHQHb as ± 0.5 g/dL and a change in tHb as ± 0.25 g/dL, the researchers found that changes in direction agreed with tHb changes in direction as follows: in 94.2% (88.9 – 97.0%) of SpHb changes, in 98.9% (96.1 – 99.7%) of ABGHb changes, and in 99.0% (96.4 – 99.7%) of aHQHb changes.
The researchers concluded, "We found that SpHb, ABGHb and aHQHb changes more than ± 0.5 g/dL have similar correlation to the direction but not necessarily the magnitude of tHb change during surgery. The similar agreement in trend direction suggests that clinicians can choose which to use based on availability or preference, although continuous SpHb monitoring may provide useful ongoing Hb trend information. Continuous noninvasive SpHb decreases exceeding −0.5 g/dL may prompt a decision to obtain a confirmatory tHb measurement if low tHb is clinically suspected, but not replace blood Hb measurement in guiding transfusion decision-making."
Comparing their results to two previous single-center studies involving changes in SpHb compared to changes in tHb,2-3 the researchers noted that their multicenter study produced “similar” results in both cases: one study (of volunteers) found 95.4% SpHb change agreement in 22 samples with tHb < 10.0 g/dL,2 while the other study (of 70 trauma patients) reported bias of -0.05, with limits of agreement of -0.62 to 0.51.3
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-9 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Applegate RL, Applegate PM, Cannesson M, Peiris P, Ladlie B, and Torp K. Multicenter comparison of three intraoperative hemoglobin trend monitoring methods. J Clin Monit Comput. 3 Dec 2019. https://doi.org/10.1007/s10877-019-00428-3.
2. Marques NR, Kramer GC, Voigt RB, Salter MG, and Kinsky MP. Trending, accuracy, and precision of noninvasive hemoglobin monitoring during human hemorrhage and fixed crystalloid bolus. Shock. 2015;44(Suppl 1):45–9. https ://doi.org/10.1097/SHK.00000 00000 00031 0.
3. Gamal M, Abdelhamid B, Zakaria D, Dayem OAE, Rady A, Fawzy M, and Hasanin A. Evaluation of noninvasive hemoglobin monitoring in trauma patients with low hemoglobin levels. Shock. 2018;49(2):150–3. https ://doi.org/10.1097/SHK.00000 00000 00094 9.
4. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
5. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
6. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
7. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
8. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
9. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
10. Estimate: Masimo data on file.
11. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SpHb® and Radical-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo SpHb and Radical-7, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Uses Masimo Patient SafetyNet™ and rainbow Acoustic Monitoring® to Investigate the Incidence of Desaturation and Bradypnea in Postoperative Patients
Neuchatel, Switzerland - November 21, 2019 – Masimo (NASDAQ: MASI) announced today that in a study recently published in the Journal of Clinical Monitoring and Computing, researchers at Nippon Medical School in Tokyo, Japan used Masimo Patient SafetyNet™ and rainbow Acoustic Monitoring® (RAM®), acoustic respiration rate monitoring with RRa®, as a centralized continuous monitoring system to identify the incidence and predictors of desaturation and bradypnea in postoperative patients – concluding that “Use of monitoring systems might provide a safety net for postoperative patients.”1
-

Masimo Patient SafetyNet™, Root® with Radius-7®, rainbow Acoustic Monitoring®, and SET® Pulse Oximetry
Patient SafetyNet is a supplemental remote monitoring, patient surveillance, and clinician notification system that works in conjunction with Masimo and third-party bedside monitoring devices to display near real-time data at central stations. RAM with RRa uses an acoustic transducer positioned on the patient’s neck to provide noninvasive, continuous respiration monitoring.
Hypothesizing that postoperative desaturation and bradypnea might occur even in non-ICU patients without serious complications, and in patients who did not undergo major surgery, Drs. Masashi Ishikawa and Atsuhiro Sakamoto set up a centralized postoperative monitoring system in the general ward to investigate how common these events are for such patients (and what might predict them). They analyzed demographic and monitoring data from 1,064 adult patients who underwent general anesthesia for various surgical procedures over a 4-month period. The patients were monitored using a pulse oximeter and an RRa sensor for at least 8 hours after surgery, data which were automatically transferred to Patient SafetyNet.From the data stored on Patient SafetyNet, the researchers were able to retrospectively analyze the incidence of desaturation (defined as SpO2 < 90% for > 10 seconds) and bradypnea (defined as respiratory rate < 8 breaths/minute for > 2 minutes). They found that 12.1% of patients exhibited desaturation (244 events among 129 patients), with most occurring after the termination of oxygen administration, and 50.8% of the events occurring more than 8 hours after surgery. They found that 5.1% of the patients exhibited bradypnea (112 times among 54 patients), with 72.3% of the events occurring during oxygen supplementation, and with the greatest incidence within the first hour after surgery. Age, body mass index, and current smoking status were significant risk factors for desaturation. Sleep apnea syndrome and postoperative opioid administration were significant risk factors for bradypnea. Age and postoperative opioid administration were significant risk factors for the combination of desaturation and bradypnea.
The researchers concluded, "Our study suggests that use of a continuous and centralized respiratory monitoring system for overnight postoperatively is desirable for postoperative management in the general ward, which would likely improve the safety of postoperative patients, especially those with risk factors for respiratory depression."
The researchers commented on a number of methods of respiratory rate monitoring, stating, "Continuous and centralized monitoring of oxygen saturation and respiratory rate can detect respiratory depression before it results in critical events such as cardiac arrest. Several methods of respiratory rate monitoring are currently used, including manual counting of breaths by a caregiver, capnography, and transthoracic impedance measurement. Manual counting of breaths (such as auscultation) is an intermittent, labor-intensive and unreliable method. Capnography provides accurate and continuous monitoring, but requires a nasal or facial interface, which can be uncomfortable and may lead to failure if the interface is moved. Transthoracic impedance is non-invasive and can detect respiratory efforts, but is unable to detect alveolar hypoventilation caused by airway obstruction."2-6
Additionally, the researchers commented on RAM with RRa, the respiratory rate monitoring method used in the current study, and referenced another study in which RRa and capnography were compared, stating: "RRa is an acoustic monitoring device that continuously measures respiratory rate, and is as accurate as capnography in extubated patients.7 Patient activities, such as talking, coughing and crying, affect the results of both RRa and capnography. The measurement errors during these activities are, however, not clinically relevant because they require that the patients are awake and breathing. Further, the RRa sensor appears to be well-tolerated and no more subject to error than capnography.7 RRa was found to be a reliable device and had fewer complications in this study."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-9 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Ishikawa M and Sakamoto A. Postoperative desaturation and bradypnea after general anesthesia in non-ICU patients: a retrospective evaluation. J Clin Monit Comput. 2 Mar 2019. https://doi.org/10.1007/s10877-019-00293-0.
2. Petterson MT, Begnoche VL, and Graybeal JM. The effect of motion on pulse oximetry and its clinical significance. Anesth Analg. 2007;105(6 Suppl):78–84.
3. Wilkinson JN, and Thanawala VU. Thoracic impedance monitoring of respiratory rate during sedation—is it safe? Anaesthesia. 2009;64(4):455–6.
4. Cohen KP, Ladd WM, Beams DM, Sheers WS, Radwin RG, Tompkins WJ, and Webster JG. Comparison of impedance and inductance ventilation sensors on adults during breathing, motion, and simulated airway obstruction. IEEE Trans Biomed Eng. 1997;44(7):555–66.
5. Drummond GB, Nimmo AF, and Elton RA. Thoracic impedance used for measuring chest wall movement in postoperative patients. Br J Anaesth. 1996;77(3):327–32.
6. Brouillette RT, Morrow AS, Weese-Mayer DE, and Hunt CE. Comparison of respiratory inductive plethysmography and thoracic impedance for apnea monitoring. J Pediatr. 1987;111(3):377–83.
7. Mimoz O, Benard T, Gaucher A, Frasca D, and Debaene B. Accuracy of respiratory rate monitoring using a non-invasive acoustic method after general anaesthesia. Br J Anaesth. 2012 May;108(5):872–5.
8. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
9. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
10. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
11. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
12. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
13. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
14. Estimate: Masimo data on file.
15. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™, RAM®, and RRa®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo Patient SafetyNet, RAM, and RRa, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces CE Marking of Radius Capnography™ for the Root® Patient Monitoring and Connectivity Platform
Radius Capnography with Bluetooth® Connectivity Seamlessly Integrates Tetherless Mainstream Capnography on Root
Orlando, Florida - October 23, 2019 – Masimo (NASDAQ: MASI) announced today that Radius Capnography™, a portable real-time capnograph with wireless Bluetooth® connectivity, has received CE marking. Radius Capnography connects with the Root® Patient Monitoring and Connectivity Platform to provide seamless, tetherless mainstream capnography for patients of all ages. Radius Capnography is the second wireless sensor created by Masimo, joining Radius PPG™, or Radius Photoplethysmography, the first tetherless sensor solution to offer Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry.
-

Masimo Root® with Radius Capnography™
Radius Capnography requires no routine calibration and minimal warm-up time, with fully accurate EtCO2 and respiration rate measurements and continuous EtCO2 waveforms displayed within 15 seconds.1 Wirelessly connected to Root, Radius Capnography presents a compelling mainstream capnography solution:
- • Cable-free Capnography: The lack of a cable tethering the Radius Capnography sensor and patient to Root improves workflow and reduces the possibility of an interruption in capnography monitoring by minimizing tugging on the breathing circuit.
- • Automated Documentation: Root automates electronic charting of patient data, including the data collected by Radius Capnography, in hospital electronic medical record (EMR) systems, working with Masimo Patient SafetyNet™ or Iris Gateway® to simplify and speed workflow, as well as reduce the likelihood of transcription errors.2
- • Maximized Data Visibility and Manipulation: Root's large, multi-touch high-resolution screen provides an easily interpretable secondary display of large, crisp EtCO2 waveforms, improving visibility and assisting clinicians in identifying wave patterns suggestive of airway obstruction or tube dislodgement. Clearly displayed trend data for up to 96 hours helps clinicians review patient progress over time, helping guide ventilation efforts. And the intuitive touch-screen interface allows clinicians to quickly adjust the trend display range and configure alarm settings to meet the needs of each patient.
- • Hassle-free Connectivity: Radius Capnography's cable-free design and quick Bluetooth pairing provide the benefits of reliable capnography and connection to Root without the burden or clutter of additional cables, facilitating easy movement between care areas, as patients move through the hospital, and in busy operating rooms where space is already at a premium.
American Heart Association guidelines recommend continuous quantitative waveform capnography, in addition to clinical assessment, to help clinicians confirm endotracheal tube placement3, assess the depth and effectiveness of chest compressions during CPR4, and recognize the return of spontaneous circulation during CPR.4
Root is a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root's plug-and-play expansion capabilities allow clinicians to simultaneously monitor with Radius Capnography and other measurements, such as Radius PPG SET® Measure-through Motion and Low Perfusion pulse oximetry and advanced rainbow® Pulse CO-Oximetry measurements, for expanded visibility of patient status.
Joe Kiani, Founder and CEO of Masimo, said, "We're happy to introduce the second tetherless, cableless sensor for Root, Radius Capnography. With this CE marking, the advanced connectivity of Radius Capnography is now available in both the US and CE-marked countries, bringing the power of Masimo capnography to even more hospitals around the world."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,6 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-10 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. EMMA Operator’s Manual.
2. The Value of Medical Device Interoperability. West Health Institute. 1013.
3. Link MS, et al. Circulation. 2015; 132(suppl 2): S444–S464.
4. Neumar RW et al. Circulation. 2010;122:S729-S767.
5. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
6. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
7. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
8. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
9. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012
10. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
11. Estimate: Masimo data on file.
12. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radius Capnography™, Root®, Patient SafetyNet™, and Iris Gateway®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo Radius Capnography, Root, Patient SafetyNet, and Iris Gateway, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Investigates Effects of Masimo SpHb®, Noninvasive and Continuous Hemoglobin Monitoring, on Postoperative Anemia and Inappropriate Blood Transfusion in Patients Undergoing Hip and Knee Surgery
Orlando, Florida - October 23, 2019 – Masimo (NASDAQ: MASI) announced today the findings of an abstract recently presented at the American Society of Anesthesiologists (ASA) 2019 Annual Meeting. In this independent study, researchers at Fukuoka University in Japan evaluated whether using Masimo SpHb® could help avoid postoperative anemia and inappropriate blood transfusion in patients undergoing total hip arthroplasty (THA) and total knee arthroplasty (TKA).1 SpHb, noninvasive, continuous hemoglobin monitoring, provides real-time visibility to changes to hemoglobin between invasive blood samples.
-

Masimo Radical-7® with SpHb®
Noting that anemia and blood transfusion are related to morbidity and mortality in surgical patients, Dr. Nakamori and colleagues sought to determine the utility of SpHb monitoring in avoiding postoperative anemia and inappropriate blood transfusion. Patients undergoing THA or TKA were randomly assigned to either an SpHb-guided group (n=73) or routine care (control) group (n=72). In both groups, patients were continuously monitored using SpHb, but in the control group clinicians were blinded to the SpHb data and blood transfusion decisions were made according to traditional clinical signs such as blood loss and hemodynamic parameters. Hemoglobin (Hb) was measured invasively using a laboratory hematology analyzer on the first postoperative day (POD1), with a target Hb value of ≥ 8 g/dL for all patients. The researchers defined two types of blood transfusion as inappropriate: excessive blood transfusion, defined as transfusion of patients with Hb > 12 g/dL, and delayed blood transfusion, defined as transfusion of patients with Hb < 8 g/dL.
The researchers found that Hb on POD1 was not significantly different between the SpHb and control groups, nor was there a significant difference in blood transfusion between both groups. However, in the control group, 7 patients received inappropriate blood transfusion (5 excessive, 2 delayed). No patients received inappropriate blood transfusion in the SpHb group (p = 0.002).
The researchers concluded, "Postoperative monitoring of SpHb cannot reduce blood transfusion nor [have an] effect on Hb at POD1, but it can prevent accidental and transient anemia, and excessive or delayed blood transfusion in patients undergoing THA and TKA."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering among other factors patient condition and laboratory diagnostic tests using blood samples. The transfusion threshold of 8 g/dL was predetermined by the study protocol and may not be appropriate for all patients.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Nakamori E, Iwashita K, Higashi M, and Yamaura K. Effect of Noninvasive Hemoglobin Monitoring on Postoperative Anemia and Inappropriate Blood Transfusion in Patients Undergoing Total Hip Arthroplasty and Total Knee Arthroplasty: A Randomized Controlled Study. Proceedings from the ASA 2019 Annual Meeting in Orlando, Florida. Abstract #A4197.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Investigates the Ability of Masimo Noninvasive, Continuous Hemoglobin (SpHb®) to Provide Earlier Indication of Anemia and the Impact of Anemia on Patient Outcomes
Neuchatel, Switzerland – September 23, 2019 – Masimo (NASDAQ: MASI) announced today that in a study published in Injury, researchers used Masimo SpHb® – noninvasive, continuous hemoglobin monitoring – to investigate possible delays in the detection of perioperative anemia assessed using invasive, intermittent laboratory hemoglobin values in elderly patients undergoing hip fracture surgery.1 In addition, they investigated associations between a) this delay and cumulative perioperative time with anemia, monitored using SpHb, and b) patient outcomes (postoperative delirium and mortality or severe complications).
-

Masimo Radical-7® with SpHb®
Dr. Christopher G. Clemmesen and colleagues at Copenhagen University Hospital in Denmark sought to investigate the impact of anemia during surgery on patient outcomes – and whether noninvasive hemoglobin monitoring using Masimo SpHb might provide an earlier indication of perioperative anemia, as well as more effectively track total time with perioperative anemia, than traditional, intermittent invasive blood sampling. Data from 41 patients, aged 65 or older, undergoing surgery to repair hip fractures, were analyzed. Blood samples were taken as per standard hospital protocol, with transfusions triggered by a hemoglobin (Hb) value below 10 g/dL, which was also used to define anemia. Throughout surgery, SpHb, as well as oxygen saturation (SpO2), perfusion index (Pi), and pulse rate (PR), were noninvasively monitored using Masimo Radical-7® Pulse CO-Oximeters and rainbow® sensors. The clinicians were blinded to data other than SpO2 to prevent them from using SpHb or Pi values to guide transfusion or other clinical decisions.
The researchers found a mean delay in the detection of anemia (defined as the time lag between an SpHb value of 10 g/dL or less and a similarly low Hb value obtained through blood sampling) of 1.07 hours (± 2.84 hours). They found a significant association between median cumulative perioperative time with low SpHb (defined as SpHb below 10 g/dL for at least one minute) and postoperative delirium: 162 minutes for patients with delirium vs. 22 minutes for patients without (p = 0.034).
The researchers concluded, “In conclusion, we found a delay in transfusion threshold detection on average, and for some patients, the delay was substantial despite the study being done in an optimized perioperative setting in a specialized ward. Continuous monitoring with SpHb during the perioperative period revealed that some patients had Hb levels below the prescribed transfusion threshold for a prolonged period. Furthermore, we found a significant association between the presence of low SpHb and postoperative outcomes, and between the cumulated time with low SpHb and postoperative outcomes... Whether increased monitoring translates into improved patient outcomes will require further studies.”
Study co-author Dr. Nicolai B. Foss commented, "There can be a significant delay in perioperative detection of anemia in hip fracture patients, and the time spent anemic, as measured continuously by SpHb, was associated with poor outcomes in our study. We need continuous monitoring in order to identify and act on anemia in a timely manner to improve patient outcomes. We believe that future transfusion studies should include improved perioperative monitoring of hemoglobin to help identify anemia."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition and laboratory diagnostic tests using blood samples
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Clemmesen CG, Palm H, and Foss NB. Delay in detection and treatment of perioperative anemia in hip fracture surgery and its impact on postoperative outcomes. Injury (2019). https://doi.org/10.1016/j.injury.2019.09.001.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Development of Delta cHb, HHb, and O2Hb Indices for O3®
Irvine, California – September 9, 2019 – Masimo (NASDAQ: MASI) announced today three additional indices (delta cHb, delta HHb, and delta O2Hb) for O3® Regional Oximetry. These indices provide clinicians with additional visibility into changes in the underlying oxyhemoglobin and deoxyhemoglobin components used to calculate cerebral oxygen saturation, rSO2. With these additions, clinicians will now be able to view the relative contribution of each component to a patient’s overall rSO2. O3, available on the Masimo Root® Patient Monitoring and Connectivity Platform, is FDA cleared for the monitoring of cerebral oxygenation and may be helpful in situations in which peripheral pulse oximetry alone may not be fully indicative of the oxygenation of the brain.
-

Masimo Root® with O3®
O3 uses near-infrared spectroscopy (NIRS) to monitor and display continuous rSO2 values for each side of the brain. As the degree of oxygenation in cerebral tissue changes, the wavelengths of light absorbed by that tissue and those returned to the O3 sensors also change, forming the basis for the measurement of regional (cerebral) oxygen saturation, rSO2. Until now, rSO2 has been displayed as a single, continuous value for each side of the brain. With these three new indices, O3 can now display information about the changes in the underlying components used to calculate rSO2 values. Delta O2Hb provides an index representing changes in the oxyhemoglobin component of the rSO2 calculation. Delta HHb provides an index representing changes in the deoxyhemoglobin component of the rSO2 calculation. Finally, delta cHb provides an index representing the sum of delta O2Hb and delta HHb.
O3 is available as a Masimo Open Connect® (MOC-9®) module for Root, a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root's plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3 and other measurements, such as SedLine® brain function monitoring – for a more complete picture of the brain – and SET® Measure-through Motion and Low Perfusion™ pulse oximetry, for expanded visibility of oxygenation status. O3 is available for all patient populations, with sensors in three sizes, for adult (≥ 40 kg), pediatric (≥ 5 kg and < 40 kg), and infant and neonatal (< 10 kg) patients.
Joe Kiani, Founder and CEO of Masimo, said, "We are proud to announce these three O3 indices, which we developed in response to requests from clinicians. Now, for the first time, clinicians can monitor not just overall cerebral oxygen saturation but also have access to additional data on the changes in the underlying oxyhemoglobin and deoxyhemoglobin components that make up rSO2 values – data that we hope can help provide additional insight into patient status."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Estimate: Masimo data on file.
8. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3® and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3 and Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Pathway™, a Newborn Oxygenation Visualization Mode for the Root® Patient Monitoring and Connectivity Platform
Irvine, California – September 4, 2019 – Masimo (NASDAQ: MASI) today announced Pathway™, a feature for the Root® Patient Monitoring and Connectivity Platform. Pathway provides clinicians with a way to visualize a hospital's recommended resuscitation protocol for a newborn's oxygen saturation (SpO2) while continuously monitoring SpO2 and pulse rate (PR) during the first ten minutes after birth. Use of Pathway is intended to help streamline clinician workflow and improve protocol adherence during this critical period. Pathway was invented by Dr. Yacov Rabi, MD, Associate Professor in the Department of Pediatrics at the Cumming School of Medicine, University of Calgary, Canada, and licensed to Masimo.
-
Masimo Root® with Pathway™
The International Liaison Committee on Resuscitation (American Heart Association) has developed treatment recommendations outlining time-based SpO2 targets that reflect a healthy newborn's oxygenation during the first ten minutes after birth.1 Current hospital practice relies on manually referencing charts or clinician recollection for adherence to protocols based on these recommendations. The ability to display local oxygen saturation targeting guidelines while still actively monitoring SpO2 and PR via Pathway's intuitive interface is intended to help clinicians more easily adhere to their institution's resuscitation protocols. While Pathway is active, Root displays SpO2 values alongside a visualization of the hospital-defined SpO2 targets over time. To help clinicians track time since birth, Pathway displays an easily adjustable timer bar so that clinicians no longer need to mentally track the difference between the time of birth and the start of monitoring. Pathway can also provide hospitals with the ability to monitor and capture data involving adherence to their chosen newborn resuscitation protocols.
Dr. Rabi commented, "We created Pathway to simplify decision-making during newborn resuscitation. I’m especially excited by the ability to review oxygen-saturation-targeting performance post-resuscitation as a means of supporting quality improvement and quality assurance initiatives at each institution."
Pathway's visualization on Root works in tandem with continuous SpO2 monitoring powered by Masimo SET® Measure-through Motion and Low Perfusion™ technology, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Crucially for newborn health, SET® has been shown to help clinicians reduce severe retinopathy of prematurity in neonates3 and in multiple studies, including the largest critical congenital heart disease (CCHD) study to date, to improve CCHD screening in newborns.4-5
Masimo Root is a powerful, versatile, and ever more expandable monitoring and connectivity hub designed for use across the continuum of care. Root integrates an array of technologies, devices, and systems to provide multimodal monitoring and automation solutions. Root's plug-and-play expansion capabilities allow clinicians to simultaneously monitor with core Masimo technologies such as SET® pulse oximetry and rainbow® Pulse CO-Oximetry while incorporating additional modalities such as SedLine® brain function monitoring, O3® regional oximetry, NomoLine® capnography, and more – all via an easy-to-interpret, customizable display that can also be set to Vital Signs Check mode and now Pathway mode. Using Root in combination with Patient SafetyNet™ or Iris Gateway™, monitoring data can be automatically charted in electronic medical records (EMRs).
Joe Kiani, Founder and CEO of Masimo, said, "From our inception, we have been committed to improving outcomes for the youngest and most fragile patients. Our foundational SET® pulse oximetry was designed with newborns and infants in mind. These patients were never an afterthought, and we continue to seek new ways to help clinicians provide them with the best care possible. One such result is Pathway, which visualizes the reliable, accurate data harnessed by SET® in an innovative and intuitive new manner. We hope that Pathway will help clinicians more efficiently and easily keep track of a newborn’s status during the critical first minutes after birth."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-8 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000267.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
6. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
7. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
8. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
9. Estimate: Masimo data on file.
10. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3® and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3 and Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Investigates the Utility of Masimo ORi™, Oxygen Reserve Index, to Reduce Hyperoxia in Critically Ill Patients
Neuchatel, Switzerland – August 26, 2019 – Masimo (NASDAQ: MASI) announced today that in a randomized controlled study involving 150 ICU patients recently published as a letter in Intensive Care Medicine, researchers investigated whether monitoring with Masimo ORi™ (Oxygen Reserve Index) could reduce the time critically ill patients spend with moderate hyperoxia, compared to monitoring with oxygen saturation (SpO2) alone.1 ORi is an index of oxygenation in the moderate hyperoxic region, defined as arterial partial pressure of oxygen, PaO2, in the range of 100 to 200 mmHg). As an “index” with a scale between 0.0 and 1.0, ORi can be trended to notify clinicians of changes in a patient’s oxygen reserve.
Dr. Sigismond Lasocki and colleagues at the Centre Hospitalier Universitaire of Angers, France (CHU Angers) sought to evaluate whether controlling the fraction of inspired oxygen (FiO2) in mechanically ventilated patients based on monitoring with ORi, rather than with SpO2 alone, could reduce the time patients spend in a moderately hyperoxic state (PaO2 ≥ 100 mmHg). 150 critically ill adult patients, all of whom were mechanically ventilated for at least 2 days, were divided randomly into an ORi and a control group. ORi and SpO2 were measured using the Masimo Root® Patient Monitoring and Connectivity Platform and rainbow® sensors. In the ORi group, nurses were instructed to decrease FiO2 when ORi ≥ 0.01. In the control (SpO2 only) group, they were instructed to decrease FiO2 when SpO2 ≥ a prescribed upper limit. PaO2 was measured by analyzing arterial blood gasses.
Data from 75 patients in the ORi group and 71 patients in the control group were analyzed. The researchers found that monitoring with ORi allowed a statistically significant reduction in the percentage of days patients spent with hyperoxia without a statistically significant increase in hypoxia: 14% were hyperoxic (0 to 33%) in the ORi group vs. 33% (11 to 56%) in the control group (p = 0.003). The percentage of hours spent with hyperoxia was also “much lower” using ORi: 7.4% were hyperoxic (0 – 24.8%) in the ORi group vs. 17.3% (3.8 – 43.1%) in the control group for PaO2 ≥ 100 mmHg (p = 0.0069), and 0% (0 – 7.2%) in the ORi group vs. 5.6% (0 – 18.1%) in the control group for PaO2 ≥ 120 mmHg (p = 0.0037).
The researchers concluded that “The use of ORi monitoring to titrate oxygen rates allowed an important reduction of the time spent with hyperoxia compared with the use of SpO2 alone, probably because nurses are reluctant to decrease oxygen rates when SpO2 is in a normal range. A nurse-driven protocol to adjust FiO2 according to SpO2 was already in place in our unit, explaining why the percentage of time with hyperoxia we observed in the control group was much less than usually reported (30 vs 60%). This strategy to decrease oxygen rate according to ORi (which detects high PaO2) may thus be even more efficient in units where there is no protocol to adjust oxygen rates. SpO2 could remain a warning for hypoxia and ORi for hyperoxia. Larger studies are needed to evaluate the clinical benefit of this strategy.”
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Lasocki S, Brochant A, Leger M, Gaillard T, Lemarié P, Gergaud S, and Dupré P. ORi monitoring allows a reduction of time with hyperoxia in critically ill patients: the randomized control ORi study. Intensive Care Med. 13 Aug 2019. https://doi.org/10.1007/s00134-019-05732-9.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Investment in Pulmonary Care Company TNI medical AG
Irvine, California – August 15, 2019 – Masimo (NASDAQ: MASI), a global leader in innovative noninvasive monitoring technologies, today announced it has closed on a strategic investment with TNI medical AG (“TNI”), a privately held, commercial-stage medical device company headquartered in Würzburg, Germany.
TNI’s novel softFlow technology is designed to provide high flow, warmed and humidified respiratory gases to spontaneously breathing patients suffering from pulmonary disease. The softFlow technology provides efficient and comfortable respiratory support by generating a precisely regulated, stable high flow of room air or a mix of room air and oxygen. The system, comprised of an integrated flow generator, respiratory circuit, and patient interface, operates without the need of pneumatic systems (i.e. compressed air supplied from the hospital wall) and can be used both in the hospital and at home.
On adult patients, continuous monitoring with SpHb has been found to improve outcomes, such as reducing the percentage of patients receiving transfusions,1 reducing the units of red blood cells transfused per patient,2-3 reducing the time to transfusion,4 reducing costs,5 and even reducing mortality 30 days after surgery.6
“The innovative products TNI has developed provide clinicians with important additional tools to address the growing number of people affected by pulmonary diseases,” said Joe Kiani, Founder, Chairman, and CEO of Masimo. “We are happy to provide TNI with an investment that will allow the company to continue to commercialize its products in the large and fast growing High Flow Nasal Therapy market.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.14-16 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,17 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.18 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
2. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
3. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
4. Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
5. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
6. Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
7. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
8. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
9. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
10. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
11. Annabi EH et al. Anesth Analg. 2009 Mar;108(3):898-9.
12. Riou Y et al. Pediatric Research. 1998. 43, 295-295.
13. U.S. Food & Drug, Consumer Updates, Benzocaine and Babies: Not a Good Mix.
14. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
15. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
16. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
17. Estimate: Masimo data on file.
18. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Investor Contact:
Masimo
Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact:
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Demonstrates Reduction in Mortality Using Masimo Noninvasive, Continuous Hemoglobin (SpHb®) and Pleth Variability Index (PVi®) Monitoring
Neuchatel, Switzerland – August 7, 2019 – Masimo (NASDAQ: MASI) announced today that in a study published in the Journal of Clinical Monitoring and Computing, researchers investigated the effects of implementing a hospital-wide fluid and blood administration protocol using two Masimo measurements: noninvasive, continuous hemoglobin (SpHb®) and pleth variability index (PVi®).1 To evaluate the impact of the implementation, they collected data on transfusions and mortality 30 and 90 days after surgery and compared the findings between two 11-month periods in 2013 and 2014.
-

Masimo Root® and Radical-7® with SpHb® and PVi®
In the study, Dr. Jérôme Cros, Prof. Nathalie Nathan, and colleagues at Hôpital Dupuytren, part of the Centre Hospitalier Universitaire of Limoges, France (CHU Limoges), sought to determine if the use of a goal-directed therapy (GDT) algorithm based on monitoring with SpHb and PVi could decrease blood requirements and reduce mortality in common clinical practice. The researchers divided 18,716 patients into 3 groups: G1 (9285 patients who underwent surgery in 2013, before implementation of the goal-directed therapy algorithm), G2 (5856 patients who underwent surgery in 2014 without use of the algorithm), and G3 (3575 patients who underwent surgery in 2014 with use of the algorithm).
For the 2014 patients, Masimo Radical-7® Pulse CO-Oximeters® equipped with SpHb and PVi were installed in all operating rooms, recovery rooms, and intensive care units. The entire anesthesiology team, including nurses, was trained on use of the monitors and the algorithm, and was free to decide whether to use goal-directed therapy for each case. Transfusion and mortality data were recorded for all patients.
Mortality Results
Using multivariate analysis and including age, ASA class, surgical severity and emergency as co-variables, the risk of death for G3 patients was 33% lower at 30 days and 29% lower at 90 days, compared to G1 patients. By contrast, there was no difference in the risk of death between G2 and G1 patients.
The authors also reported on mortality rate the year after the study ended (2015), when the hospital no longer had access to SpHb and PVi. Comparing 2015 patients to patients in the study, they found that mortality at 30 and 90 days increased again to levels similar to those found in 2013 (before implementation), respectively 2.18% and 3.09%.
The authors noted, "Because patients who did not receive GDT based on the PVi had similar mortality rates in 2014 and 2013, a Hawthorne effect-inducing care improvement does not explain the present results. The post-study increase in mortality, at the time when monitors were no longer available, suggests that education of the team to improve fluid management does not explain the present results."
Transfusion Results
After adjusting for surgical severity, age, and ASA class, patients in G3 had reduced odds of being transfused within 48 hours (odds ratio of 0.79, 95% CI of 0.68 – 0.93, p = 0.004). By contrast, there was no difference in the odds of being transfused between patients in G2 and G1.
The authors noted, "This study shows that using an algorithm based on continuous Hb measurement and fluid responsiveness with PVi in common clinical practice is associated with different transfusion practices and a lower adjusted-mortality at 1 and 3 months. When considering confounding factors such as ASA class, severity of surgery and emergency, the monitor-based algorithm lowers transfusion probability by approximately 30% during surgery and at 48 h. In non-cardiac surgeries, patients were transfused sooner and more often but with less blood units in the GDT group. In non-cardiac surgery, continuous Hb monitoring alerted anesthesiologist[s] on the anemia risk they might [have] under-evaluated without monitoring. This was the opposite, in cardiac surgery where practitioners behave differently. When using continuous SpHb monitoring, perioperative transfusion was reduced because anesthesiologists probably less feared under-transfusion. The net observed effect was an 11% and 6.5% reduction in blood units transfused in the operating room and at 48 h."
The researchers concluded, "Monitoring SpHb and PVi integrated in a vascular filling algorithm is associated with earlier transfusion and reduced 30 and 90-day mortality on a whole hospital scale." They continued, "In conclusion, this integrated comparative effectiveness study shows that using an algorithm of fluid and blood transfusions based on continuous Hb measurement and PVi is associated with reduced mortality."
Joe Kiani, Founder and CEO of Masimo, commented, "We thank Professor Nathan and her team for this outstanding study. All of the outcome studies to date with continuous SpHb have shown its benefits in transfusion management2-5 and numerous studies with PVi have demonstrated its role in fluid management,6-7 but this is the first time a study has shown how using goal-directed therapy with SpHb and PVi can have such a big impact on mortality. As it is central to our mission, we encourage researchers to continue to study the impact of SpHb and PVi to see if indeed these fantastic results can be repeated in other institutions, for example those with different mortality rates, and if so, help expand their use to improve patient outcomes around the world."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.8 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,9 improve CCHD screening in newborns,10 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.11-13 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,14 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.15 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Cros J, Dalmay F, Yonnet S, Charpeniter M, Tran-Van-Ho J, Renaudeau F, Drouet A, Guilbaut P, Marin B, and Nathan N. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
2. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
3. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
4. Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
5. Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
6. Forget P et al. Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management. Anesth Analg. 2010; 111(4):910-4.
7. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs After Colorectal Surgery. J Am Coll Surg. 2015. Doi: 10.1016/j.jamcollsurg.2014.12.042.
8. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
9. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
10. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
11. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
12. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
13. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
14. Estimate: Masimo data on file.
15. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces CE Marking of Neonatal Indication for Noninvasive, Continuous Hemoglobin Monitoring (SpHb®)
Neuchatel, Switzerland – August 5, 2019 – Masimo (NASDAQ: MASI) announced today the CE marking of SpHb®, noninvasive and continuous hemoglobin monitoring, for neonatal and infant patients (< 3 kg). With this clearance, the benefits of SpHb are available for patients of all ages in CE mark countries. SpHb for neonates and infants is provided on rainbow® sensors, which allow clinicians to simultaneously measure multiple additional noninvasive parameters alongside SpHb, including oxygen saturation (SpO2) and methemoglobin (SpMet®).
-
 Masimo Root® with SpHb®and PVi®
Masimo Root® with SpHb®and PVi®
Because of their small size, neonates and infants have less blood than older patients. In addition, their lack of bone marrow density makes them far less capable than adults of generating new red blood cells for approximately the first eight months of life. Current invasive methodologies for measuring hemoglobin can only provide intermittent, delayed results. By providing a continuous, noninvasive measurement, SpHb allows clinicians to more closely monitor neonatal hemoglobin status in real time by tracking the stability, or instability, of a patient’s hemoglobin trend, providing visibility into changes, or lack of changes, in hemoglobin between invasive blood samples.
On adult patients, continuous monitoring with SpHb has been found to improve outcomes, such as reducing the percentage of patients receiving transfusions,1 reducing the units of red blood cells transfused per patient,2-3 reducing the time to transfusion,4 reducing costs,5 and even reducing mortality 30 days after surgery.6
In addition to hemoglobin, rainbow® sensors monitor SpO2 using Masimo SET® Measure-through Motion and Low Perfusion™ technology, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Crucially for newborn health, SET® has been shown to help clinicians reduce severe retinopathy of prematurity in neonates8 and in multiple studies, including the largest critical congenital heart disease (CCHD) study to date, to improve CCHD screening in newborns.9-10
The rainbow® family of advanced noninvasive measurements also allows clinicians to use the same single sensor to monitor another physiological parameter particularly important to neonatal care: methemoglobin, using SpMet. SpMet helps clinicians noninvasively and continuously monitor methemoglobin levels in the blood.11 In neonates and infants, inhaled nitric oxide (iNO) therapy and even topical anesthetics containing benzocaine or prilocaine can cause elevated levels of methemoglobin.12-13
Joe Kiani, Founder and CEO of Masimo, said, "We are thrilled to be able to bring the power of noninvasive hemoglobin monitoring with SpHb to the youngest, most fragile patients of all. We have long been dedicated to helping improve the lives of neonatal and infant patients, and this latest clearance significantly furthers that mission. SpHb is already used to monitor adult patients in more than 75 countries. We look forward to witnessing the impact that SpHb will now be able to have on neonatal and infant patients."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Noninvasive, continuous SpHb has received FDA clearance for patients > 3 kg but is not currently indicated for patients < 3 kg in the US.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.14-16 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,17 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.18 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
2. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
3. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
4. Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
5. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
6. Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
7. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
8. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
9. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
10. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
11. Annabi EH et al. Anesth Analg. 2009 Mar;108(3):898-9.
12. Riou Y et al. Pediatric Research. 1998. 43, 295-295.
13. U.S. Food & Drug, Consumer Updates, Benzocaine and Babies: Not a Good Mix.
14. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
15. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
16. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
17. Estimate: Masimo data on file.
18. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Reports Second Quarter 2019 Financial Results
Q2 2019 Highlights
- • Total revenue, including royalty and other revenue, was $229.7 million;
- • Product revenue increased 13.6% to $229.5 million, or 14.8% on a constant currency basis;
- • GAAP net income per diluted share was $0.79; and
- • Non-GAAP net income per diluted share increased 24.6% to $0.76.
Irvine, California, July 31, 2019 - Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended June 29, 2019.
Second Quarter 2019 Results:
Total revenue, including royalty and other revenue, was $229.7 million. Product revenue increased 13.6% to $229.5 million, or 14.8% on a constant currency basis. Shipments of noninvasive technology boards and monitors increased approximately 2.9% to 60,400 in the second quarter of 2019, compared to 58,700 in the second quarter of 2018.
GAAP operating margin was 22.6%. Non-GAAP operating margin increased 200 basis points to 23.1%, compared to 21.1% in the prior year period.
For the second quarter of 2019, GAAP net income was $44.9 million or $0.79 per diluted share. Non-GAAP net income was $43.1 million or $0.76 per diluted share, a 24.6% increase compared to $0.61 per diluted share in the prior year period.
The Company repurchased approximately 208,000 shares of Masimo common stock for a total cost of approximately $27.9 million during the second quarter of 2019. Total cash and short-term investments was $588.8 million as of June 29, 2019.
As a result of the strong performance in the second quarter, Masimo is again raising its guidance for fiscal year 2019. The Company now expects product revenue of $925.0 million, which reflects reported growth of 11.5% and constant currency growth of 12.2%. Masimo is also raising its GAAP EPS guidance to $3.30 and its non-GAAP EPS guidance to $3.15.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “The first half of 2019 has been strong with second quarter constant currency product revenue growth of 14.8% and non-GAAP earnings per share growth of 25%. We are happy to report second quarter results that once again exceeded expectations. Our breakthrough technologies and solutions improve patient outcomes and reduce cost of care. In the first half of the year we introduced six important products, including Radius™ PPG, our Halo ION™, and a neonatal version of our O3® Cerebral Oximetry monitor, all of which should help fuel our momentum. With these new products added to our existing portfolio and ever increasing global footprint, we are delighted to be in a position to raise our revenue and earnings guidance for 2019.”
2019 Financial Guidance
The Company provided the following updated estimates for its full year 2019 guidance:

- • Total revenue, including royalty and other revenue, increasing to $926.3 million;
- • Product revenue increasing 11.5% to $925.0 million, or 12.2% on a constant currency basis;
- • GAAP diluted earnings per share increasing to $3.30;
- • Non-GAAP diluted earnings per share increasing 18.9% to $3.15; and
- • Included in our full year revenue guidance is approximately $6.0 million of year-over-year currency headwinds.
Impact of Adoption of New Lease Accounting Standard
Effective December 30, 2018, we adopted Accounting Standards Codification (ASC) Topic 842, Leases (ASC 842). Our adoption of ASC 842 generally resulted in (a) the recognition of lessee right-of-use (ROU) assets for the right to use assets subject to operating leases; (b) the recognition of lessee lease liabilities for our obligation to make payments under operating leases; and (c) the acceleration of when we recognize certain revenue and costs as a lessor of equipment provided to end-user hospitals at no up-front charge under deferred equipment agreements with fixed multi-year sensor purchase commitments. For additional information with respect to the impact of the adoption of this new accounting standard, please reference Note 2 to our condensed consolidated financial statements that will be included in Part I, Item 1 of our Quarterly Report on Form 10-Q for the quarter ended June 29, 2019, once filed with the Securities and Exchange Commission (SEC) and Exhibit 99.3 that was included in our Current Report on Form 8-K that was filed with the SEC today.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s ongoing core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an ongoing basis: (i) constant currency product revenue growth %, (ii) non-GAAP net income, (iii) non-GAAP diluted earnings per share, (iv) non-GAAP gross profit/margin, (v) non-GAAP operating income/margin, (vi) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes constant currency product revenue growth, non-GAAP gross profit/margin, non-GAAP operating income/margin, non-GAAP net income, non-GAAP diluted earnings per share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency adjustments.
Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. These foreign currency revenues, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating our product revenue growth rates on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and the related constant currency adjustments for calculation of our product revenue growth rate will continue to occur in future periods.
Royalty and other revenue, net of related costs.
We derive royalty and other revenue, net of related costs, from certain non-recurring contractual arrangements that we do not expect to continue in the future. We believe the exclusion of royalty and other revenue, net of related costs, associated with these non-recurring revenue streams is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis.
Acquisition/strategic investment related costs, including depreciation and amortization.
In the event the Company acquires, invests in or divests certain business operations, there may be non-recurring gains, losses or expenses that will be recognized related to the assets and/or liabilities sold or acquired that are not representative of normal ongoing cash flows. Furthermore, there may be depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition/strategic investment that does not reflect the normal ongoing costs of operating our core business. We believe that exclusion of these gains, losses or costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds. We believe that exclusion of these gains and losses is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
From time-to-time, we may experience significant non-recurring tax events, such as changes in tax laws and regulations or the derecognition of uncertain tax positions related to non-recurring transactions due to the expiration of the statutes of limitations. We believe that exclusion of such tax charges or benefits is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these tax items are unrelated to our core business and generally unique and non-recurring in nature.
Adjusted EBITDA.
The Company defines adjusted EBITDA as earnings before non-operating income/expense, taxes, depreciation and amortization, as adjusted for the applicable non-GAAP adjustments described above and further excluding non-cash stock based compensation expense. We believe these adjustments to EBITDA provide investors and management with a more consistent measurement of business performance and aid in comparability from period to period.
Second Quarter 2019 Actuals versus Second Quarter 2018 Actuals
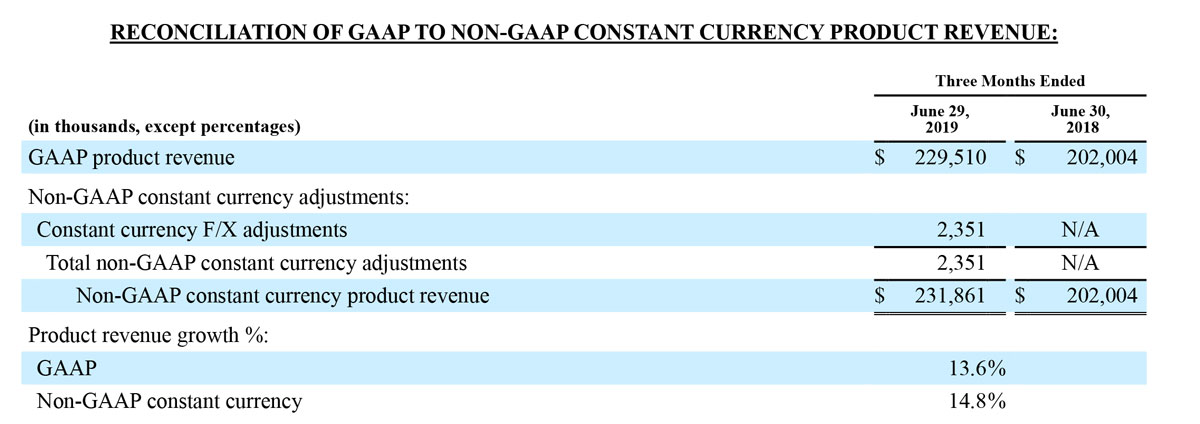
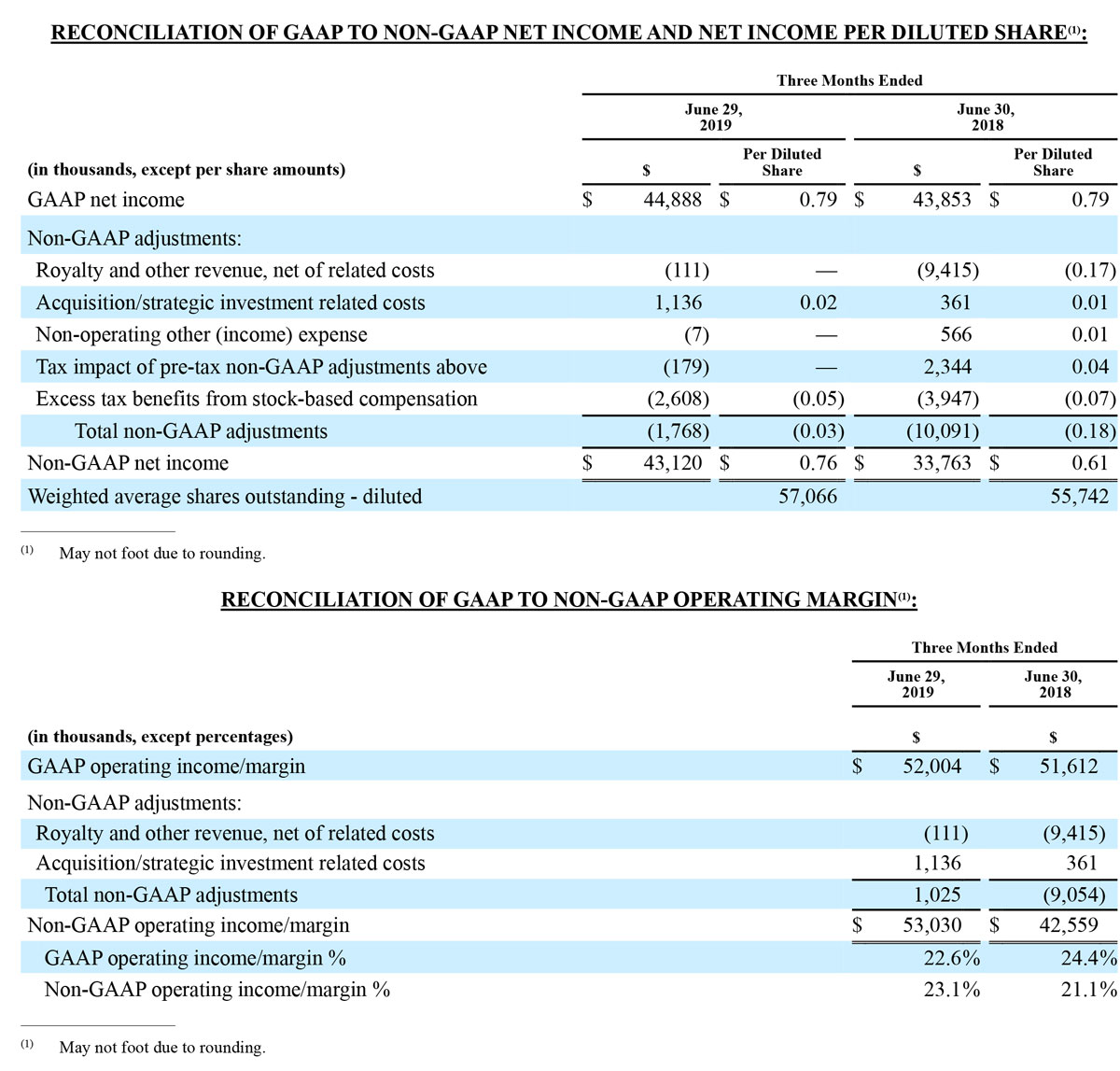

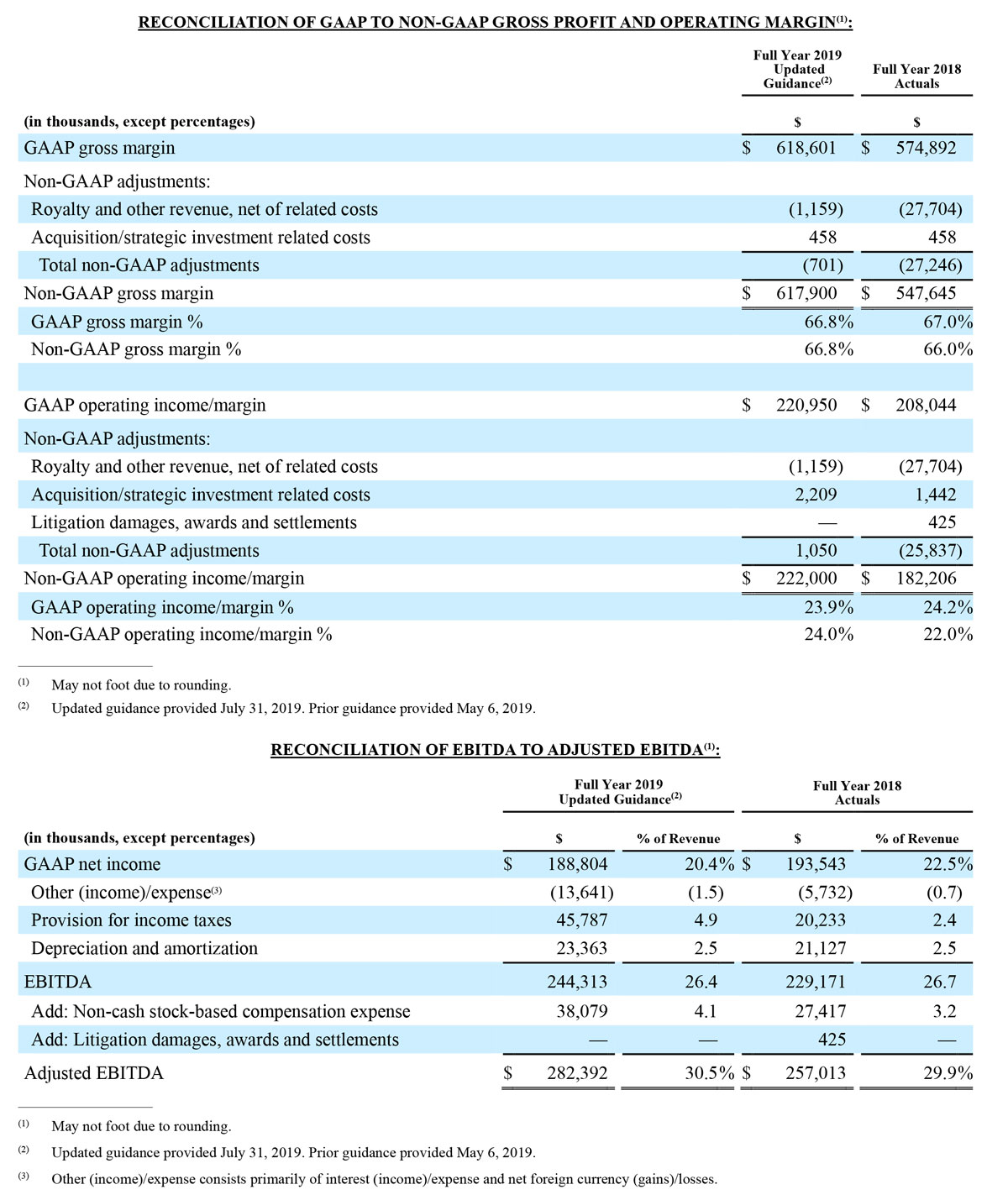
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 3195569. After the live webcast, the call will be available on Masimo’s website through August 7, 2019. In addition, a telephonic replay of the call will be available through August 30, 2019. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 3195569.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2019 total revenue, product revenue, royalty and other revenues, gross margin, operating margin, diluted earnings per share, EBITDA, estimated tax rate and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact: Irene Paigah
Phone: (858) 859-7001
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.

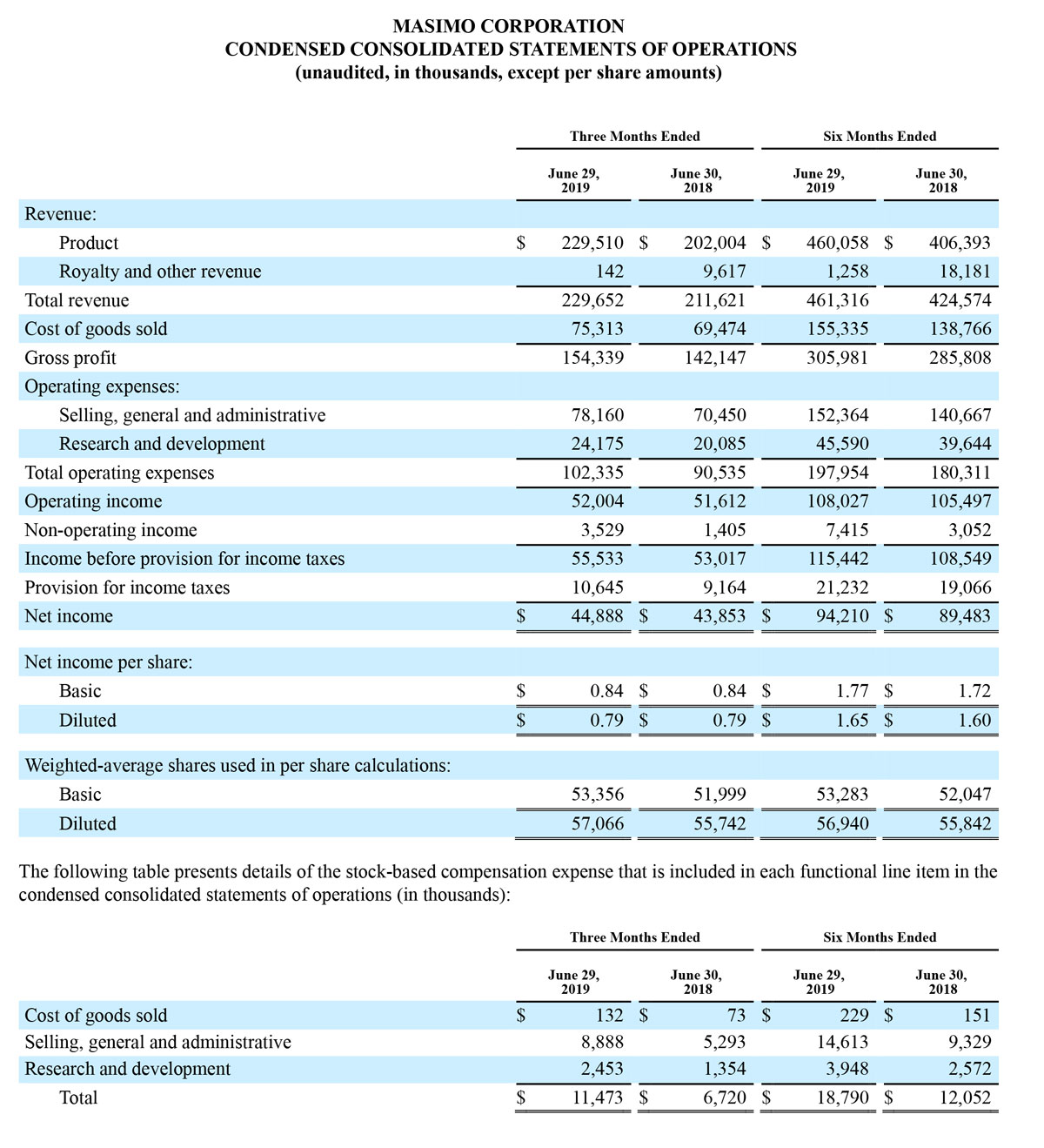
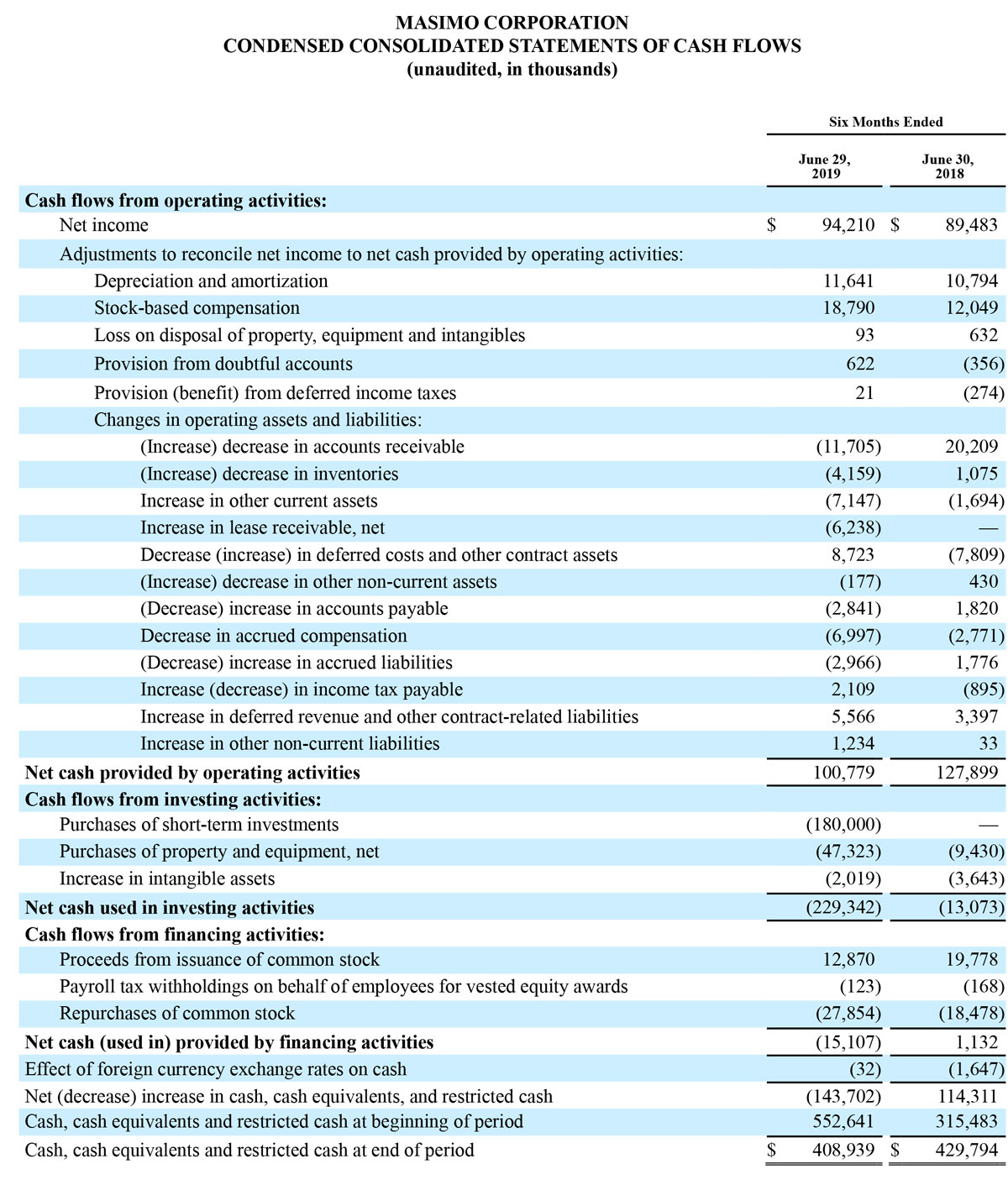

Study Investigates the Ability of Continuous Noninvasive Hemoglobin Monitoring with Masimo SpHb® to Reflect Acute Hemodilution During Incremental Fluid Administration
Neuchatel, Switzerland – July 22, 2019 – Masimo (NASDAQ: MASI) announced today that in a study recently published in Anesthesia & Analgesia, researchers evaluated the ability of noninvasive and continuous hemoglobin monitoring with Masimo SpHb® to detect the development of acute hemodilution after graded fluid administration, by comparing it to invasively measured laboratory hemoglobin (BHb), on patients undergoing major surgery.1
-

Masimo Root® with SpHb®and PVi®
In the study, Dr. Şerban Bubenek-Turconi and colleagues at the Carol Davila University of Medicine and Pharmacy and the Prof. C.C. Iliescu Institute for Cardiovascular Diseases in Bucharest, Romania, and Sheba Medical Center in Tel Aviv, Israel, examined the effects of incremental fluid loading (as part of perioperative goal-directed therapy (GDT)) on oxygen delivery and whether noninvasive SpHb monitoring could reliably track the development of acute hemodilution (which can necessitate blood transfusions that might otherwise be avoided). They analyzed data from 40 adult patients undergoing major gastrointestinal or vascular surgery. SpHb was continuously measured using Masimo Root® with the Radical-7® Pulse CO-Oximeter®. BHb and partial pressure of oxygen (PaO2) were intermittently, invasively measured using a Radiometer ABL800 blood gas analyzer. Cardiac output (CO) and stroke volume (SV) were also obtained through invasive modalities. Oxygen delivery (DO2) was calculated as: CO*((Hb*1.38*SpO2)+PaO2*0.0031)). Parameter values were recorded after the induction of general anesthesia and before the start of surgery (T0) and 5 minutes after successive 250 ml colloid fluid challenges (FC) (T1, T2, and T3). Patients were given the second and third fluid challenges if at each stage SV increased by at least 10%. 40 patients received the first FC, 32 received the second, and 20 received the third, for a total of 92 administered FCs.
The researchers found that, "Compared to their baseline values (T0), BHb and SpHb decreased by a mean of 5.3% ± 4.9% and 4.4% ± 5.2%, respectively, after the first FC (T1; n = 40), by 9.7% ± 8.4% and 7.9% ± 6.9% after the second FC (T2; n = 32), and by 14.5% ± 6.2% and 14.6% ± 5.7% after the third FC (T3; n = 20)." Using Bland-Altman analysis of all 132 paired SpHb and BHb values, the researchers found mean bias and precision values of -0.3 ± 1.5 g/dL and limits of agreement of -2.7 to 3.3 g/dL. They also found that concordance rates between changes in SpHb and in BHb after the administration of the 250, 500, and 750 mL of fluid were 83%, 90%, and 100%, respectively. They noted that results showed "excellent" concordance after 750 mL, despite the limited absolute accuracy, which may have been negatively affected by the "well-recognized inherent variability of the laboratory Hb device that served as our reference method."
The researchers concluded, "In summary, our study shows that acute iatrogenic hemodilution does invariably occur after fluid administration, that it may lead to paradoxical decrease in DO2 especially in 'nonresponders' and that its development is made visible by the continuous monitoring of SpHb when >500 mL of colloids are being administered. Further studies are needed to examine the potential clinical benefit of SpHb monitoring in identifying acute hemodilution."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Bubenek-Turconi S, Văleanu L, Popescu M, Panaitescu E, Tomescu D, Cacoveanu M, and Perel A. Continuous Noninvasive Hemoglobin Monitoring Reflects the Development of Acute Hemodilution After Consecutive Fluid Challenges. Anesth Analg. 2019. DOI: 10.1213/ANE.0000000000004323.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Investigates the Utility of Masimo ORi™, Oxygen Reserve Index, As an Indicator to Avoid Hyperoxia During General Anesthesia
Neuchatel, Switzerland – July 15, 2019 – Masimo (NASDAQ: MASI) announced today that in a study recently published in the Journal of Clinical Monitoring and Computing, researchers investigated the ability of Masimo ORi™ (Oxygen Reserve Index) to serve as a noninvasive indicator of the arterial partial pressure of oxygen (PaO2) during general anesthesia, to help avoid hyperoxia.1 ORi is an index of oxygenation in the moderate hyperoxic region (PaO2 range of 100 to 200 mmHg). As an "index" with a scale between 0.0 and 1.0, ORi can be trended to notify clinicians of changes in a patient’s oxygen reserve.
-

Masimo Root® with SedLine®
Dr. Keisuke Yoshida and colleagues at the Fukushima Medical University School of Medicine in Japan sought to evaluate whether ORi could provide continuous, noninvasive insight into avoiding excessive hyperoxia by comparing the relationship between PaO2 and ORi during various oxygen administration conditions. They enrolled 20 patients scheduled for surgery requiring general anesthesia. ORi was measured using Masimo Root® with the Radical-7® Pulse CO-Oximeter® and rainbow® sensors. PaO2 was measured using the Siemens RAPIDLab® 1265 blood gas analyzer. For each patient, after inducing anesthesia, blood gas analysis to measure PaO2 was performed four times, with ORi values recorded each time blood was drawn, providing 80 data sets. Initial analysis was performed with inspired oxygen concentration (FiO2) set to 0.33, with the three subsequent analyses performed when ORi was around 0.5, 0.2, and 0, achieved by adjusting FiO2.
For analysis, the researchers chose an upper PaO2 threshold of 240 mmHg, based on a previous study that found a positive correlation between ORi and PaO2 when PaO2 < 240 mmHg.2 They defined hyperoxemia as PaO2 ≥ 150 mmHg. Using linear regression analysis, the researchers found a “relatively strong” positive correlation (r2 = 0.706) between ORi and PaO2 when PaO2 was less than 240 mmHg. Using receiver operating characteristic (ROC) curve analysis, they calculated that the optimal cut-off ORi value to detect PaO2 ≥ 150 mmHg was 0.21 (sensitivity 0.950, specificity 0.755). Using four-quadrant plot analysis, they found that ORi trended PaO2 with a 100% concordance rate.
The researchers concluded that “Hyperoxemia can be detected by observing ORi of patients under general anesthesia, and thus unnecessary administration of high concentration oxygen can possibly be avoided.” They also noted, “ORi has unique characteristics reflecting the state of oxygenation in the hyperoxic range. The present study examined data under general anesthesia in the operating room, but ORi’s unique features can provide benefits not only in the operating room, but also in the ICU and other fields. In the future, in order to make the most use of ORi, further clinical study is required.”
As study limitations, the researchers noted the small number of subjects in the study. In addition, they noted, “It is plausible that a given patient’s factors (e.g. age, physique, body temperature, finger perfusion, hemoglobin concentration) affect their ORi value. Therefore, ORi might not reflect all changes in PaO2.”
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Yuan I, Landis W, Topjian A, Abend N, Lang S, Huh J, Kirschen M, Mensinger J, Zhang B, and Kurth C. Prevalence of Isoelectric Electroencephalography Events in Infants and Young Children Undergoing General Anesthesia. Anesth Analg. 2019. DOI: 10.1213/ANE.0000000000004221
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Uses Masimo SedLine® Brain Function Monitoring to Assess the Prevalence of Isoelectric EEG Events in Infants and Young Children Undergoing General Anesthesia
Irvine, California – June 24, 2019 – Masimo (NASDAQ: MASI) announced today that in a study recently published in Anesthesia & Analgesia, researchers investigated the incidence of isoelectric electroencephalogram (EEG) events in infants and young children undergoing scheduled surgery requiring general anesthesia.1 To assess the prevalence of these events, the researchers used Masimo SedLine® brain function monitoring, available on the Root® Patient Monitoring and Connectivity Platform, which uses four leads and bilateral data acquisition to process frontal EEG signals. Because a subset of patients was also monitored with 10-channel EEG, the researchers were able to evaluate SedLine’s accuracy and utility in such a scenario.
-

Masimo Root® with SedLine®
Dr. Ian Yuan and colleagues at the Children's Hospital of Philadelphia, the University of Pennsylvania, and Drexel University sought to evaluate the incidence of isoelectric EEG events in pediatric patients because anesthetic doses may be "greater than needed for surgery" in some infants and young children and isoelectric EEG events are associated with deep anesthesia (and in adults undergoing cardiac surgery, with "a higher incidence of postoperative delirium"). They evaluated 51 patients from 0 to 37 months old scheduled for surgery requiring general anesthesia (excluding cardiac, intracranial, and emergency cases). Anesthesia was maintained with sevoflurane or propofol infusion.
Noting that “Conventional 10- to 24-channel EEG is impractical for pediatric anesthesia as it is labor intensive and requires special training in both performance and interpretation,” the researchers chose to record EEG for the duration of anesthesia using Masimo SedLine. To evaluate whether isoelectric events were generalized beyond the frontal cortex, they also recorded simultaneous data using 10-electrode EEG (Natus Xltek) for 10 patients (2-10 months old). Isoelectric events were defined as EEG with amplitude <20 μV for ≥2 seconds.
The researchers found that isoelectric events occurred in 63% of patients (95% confidence interval of 49% – 76%), representing 0% – 2.2% of total anesthetic time. A pediatric electroencephalographer compared the EEG recordings for 9 of the 10 patients monitored with both SedLine and 10-channel EEG and found that isoelectric events observed with SedLine were also observed across all channels with 10-channel EEG, noting "The Matlab program identified 47 isoelectric events from the SedLine EEG, and all events were confirmed by the electroencephalographer."
Dr. Yuan commented, "We were surprised by how prevalent isoelectric EEG occurred in healthy infants and young children undergoing routine general anesthesia, regardless of anesthetic technique. The Masimo SedLine EEG could be applied quickly without interfering with anesthesia care and could accurately record EEG in infants and young children in the operating room, suggesting it could be used to adjust anesthesia dosing."
Study co-author Dr. C. Dean Kurth added, "Pediatric anesthesiologists often unknowingly administer more sevoflurane or propofol anesthesia to infants and young children than their brain requires to produce anesthesia."
Regarding the use of SedLine, the researchers concluded, "Although the SedLine EEG monitors only frontal cortex, the 10-electrode EEG showed that the isoelectric events were generalized to the parietal, temporal, and occipital cortices as well, suggesting that the SedLine EEG could be used to monitor anesthetic-related isoelectric events." They continued, "EEG monitoring may enable dose titration to optimize the anesthetic depth and minimize periods of isoelectricity. Our results suggest that portable 4-channel EEG monitors are feasible for this purpose." With SedLine, periods of isoelectricity, or burst suppression, are easily visible on the Density Spectral Array (DSA), a color-coded display of processed EEG power, as black bars.
SedLine and Next Generation SedLine have received FDA clearance for use on adult patients. Next Generation SedLine has received CE marking for use on pediatric patients (one-year-old and above).
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Yuan I, Landis W, Topjian A, Abend N, Lang S, Huh J, Kirschen M, Mensinger J, Zhang B, and Kurth C. Prevalence of Isoelectric Electroencephalography Events in Infants and Young Children Undergoing General Anesthesia. Anesth Analg. 2019. DOI: 10.1213/ANE.0000000000004221
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of Neonatal Indication for O3® Regional Oximetry
Irvine, California – June 10, 2019 – Masimo (NASDAQ: MASI) announced today that O3® Regional Oximetry has received FDA clearance for use on neonatal and infant patients (<10 kg). O3 may help clinicians monitor cerebral oxygenation in situations in which peripheral pulse oximetry alone may not be fully indicative of the oxygen in the brain. With this expansion of the O3 platform, the benefits of monitoring with O3 are now available to patients of all ages, from neonates to adults.
-

Masimo Root® with O3® Regional Oximetry
O3, which uses near-infrared spectroscopy (NIRS) to monitor the regional hemoglobin oxygen saturation of blood (rSO2) on both sides of the brain, may be particularly helpful in providing insight into neonatal patient status because neonatal pathology is often brain-related.1 In neonates, O3 provides reliable measurement with a 3% ARMS* trending accuracy specification.
O3 sensors are available in three sizes, for adult (≥40 kg), pediatric (≥5 kg and <40 kg) and now infant and neonatal (<10 kg) patients. With its smaller size and flexible design, the neonatal sensor is designed to fit easily and comfortably on the delicate foreheads of tiny patients.
O3 is available as a Masimo Open Connect® (MOC-9®) module for the Root® Patient Monitoring and Connectivity Platform. Root is a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root's plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3 and other measurements, such as SET® Measure-through Motion and Low Perfusion™ pulse oximetry, providing clinicians with expanded visibility of neonatal oxygenation status. Additional modalities available on Root include advanced rainbow® noninvasive measurements such as total hemoglobin (SpHb®), SedLine® brain function monitoring (available for adult patients), NomoLine® capnography, and more – all via an easy-to-interpret, customizable display. Using Root in combination with Masimo Patient SafetyNet™ or Iris Gateway™, monitoring data from O3 can be automatically charted in electronic medical records (EMRs).
Joe Kiani, Founder and CEO of Masimo, said, "From our inception, we have been committed to improving outcomes for the youngest and most fragile patients. Our foundational SET® pulse oximetry was designed with neonates and infants in mind. These patients were not an afterthought. This focus has paid off for these young patients: SET® pulse oximetry has helped clinicians reduce the incidence of severe retinopathy of prematurity (ROP) in neonates2 and improve critical congenital heart disease (CCHD) screening in newborns.3 O3 Regional Oximetry, with its ability to help clinicians accurately track cerebral oxygen saturation, will hopefully have a similar impact. We are happy to be able to bring O3's advanced capabilities and accuracy to the neonatal patient population."
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Dix et al. Monitoring Cerebral Oxygenation in Neonates: An Update. Frontiers in Pediatrics. 2017.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

NuVision Management Healthcare Consulting Company, One of the Largest U.S. Providers of Long-term Care for Medically Complex Children, Converts to Masimo
NuVision to Deploy Masimo SET®, Root® with Radical-7®, NomoLine® Capnography, and Patient SafetyNet™ at Four Locations
Fort Lauderdale, Florida – May 28, 2019 – Masimo (NASDAQ: MASI) announced today that NuVision Management Healthcare Consulting Company, a pediatric and adult healthcare consulting company, is adopting a comprehensive variety of Masimo technologies at its three pediatric care facilities in New Jersey and Florida, including Voorhees Pediatric (121 beds), Weisman Children's Rehabilitation Hospital (18 beds), and Plantation Kidz Korner (95 beds), as well as at a fourth, adult-care location, Clark Nursing and Rehabilitation Center (144 beds).
-

Masimo Root® Patient Monitoring and Connectivity Platform
Pat Budo, Director of the Pediatric Complex Care Association (PCCA), said, "NuVision is the latest member of the Pediatric Complex Care Association to install Masimo technology. They join many of current PCCA members in utilizing this technology to support children with medical complexity. Both NuVision and Masimo share our vision of promoting excellence across the continuum of care for these extraordinary children."
At every medically complex patient's bedside at the four locations, NuVision is installing Masimo Root® Patient Monitoring and Connectivity Hubs equipped with Radical-7® Pulse CO-Oximeters®, allowing patients to be monitored using Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, in addition to a variety of other rainbow SET™ parameters. NomoLine® capnography, available as a MOC-9® expansion module for Root, will be used to support the management of children on ventilators. Data from the ventilators will also be available on Root, via Masimo Iris® third-party connectivity, an integration NuVision is planning to implement this summer.
Masimo Patient SafetyNet™, a supplemental remote monitoring and clinician notification system, will allow clinicians to view monitoring data for all beds from central view stations. When changes occur in measured values that may indicate deterioration in a patient’s condition, Patient SafetyNet automatically sends wireless alerts directly to clinicians, wherever they may be, allowing them to respond quickly and efficiently to potential changes in patient status. Facilitated by Patient SafetyNet, all of the data captured by Root and devices connected to it will be transferred automatically to patient electronic medical records (EMRs) – including monitoring data, vital signs, and clinician-initiated early warning scores using the PEWS (pediatric early warning score) protocol, which NuVision plans to start using later this year as another way to help clinicians identify changes in patient condition.
"These systems and technologies have been a great addition to our organizations," said Trish Cunningham, RRT, Clinical Director of Respiratory Care at Voorhees Pediatric Facility and Weisman Children’s Rehabilitation Hospital. "We are already noticing improvements in patient care and are excited about the upcoming integrations and capabilities."
Masimo Root is a powerful, expandable bedside platform that integrates an array of technologies, devices (such as Radical-7 and the wearable Radius-7®), and systems to provide multimodal monitoring and connectivity solutions – in a single, clinician-centric hub. Root's plug-and-play expansion capabilities allow clinicians to simplify patient monitoring by bringing together advanced rainbow SET Pulse CO-Oximetry, brain function monitoring, regional oximetry, capnography, third-party devices, and vital signs measurements on an easy-to-interpret, customizable display, empowering clinicians with important information for making patient assessments.
Mike Rosiak, Chief Operating Officer of Weisman Children's Rehabilitation Hospital and Voorhees Pediatric Facility said, "Masimo's Root and Patient SafetyNet medical systems will help us to provide the best care for our medically fragile children and enable them to reach their full potential."
Jon Coleman, President of Worldwide Sales, Professional Services, and Medical Affairs, Masimo, said, "We are proud to partner with NuVision and bring the benefits of our innovative, versatile, and proven medical technologies and connectivity solutions to these particularly vulnerable patients. We look forward to helping clinicians improve patient outcomes and the lives of families with medically fragile children."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius™ PPG, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Estimate: Masimo data on file.
8. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®, rainbow SET™, Root® with Radical-7®, NomoLine®, and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, rainbow SET, Root with Radical-7, NomoLine, and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Evaluates Ability of Masimo SpHb® (Noninvasive Hemoglobin) to Estimate Timing for Invasive Measurement to Detect Anemia During Surgery
Neuchatel, Switzerland – May 20, 2019 – Masimo (NASDAQ: MASI) announced today the results of a study published in BMC Anesthesiology in which clinicians at Peking Union Medical College in Beijing evaluated the ability of noninvasive and continuous hemoglobin monitoring with Masimo SpHb® to help clinicians estimate when to conduct invasive hemoglobin measurement to detect possible anemia in patients undergoing spine or cytoreductive surgery.1
-

Masimo Radical-7® with SpHb®
Noting that because of the "invasive, time-consuming and intermittent" nature of invasive blood sampling, clinicians often forego these "objective indications" when making transfusion decisions during surgery, Dr. Tang and colleagues sought to determine whether noninvasive, continuous hemoglobin monitoring could aid clinicians in estimating when it might be appropriate to perform an invasive measurement. They enrolled 69 adult patients scheduled for spine surgery or cytoreductive surgery for whom estimated blood loss was more than 15% of total blood volume. The patients were randomly divided into an SpHb group (32 patients) and a standard care group (37 patients). In the SpHb group, diagnostic blood samples were drawn when a patient’s SpHb, measured using a Masimo Radical-7® Pulse CO-Oximeter®, decreased by 1 g/dL. In the standard care group, they were drawn at the clinicians' discretion. Blood gas analysis was performed using a Radiometer ABL800. The researchers determined the positive predictive value (PPV) of SpHb for the SpHb group and clinician perception in the standard care group in detecting a decrease in lab hemoglobin of more than 1 g/dL or lab hemoglobin of less than 10 g/dL.
The researchers found that the incidence of unnecessary hemoglobin measurement was lower in the SpHb group than the standard care group. For a decrease of greater than 1 g/dL in lab hemoglobin, SpHb had a PPV of 93.3%, compared to 54.5% for clinical perception (p = 0.002). For hemoglobin lower than 10 g/dL, SpHb had a PPV of 86.7%, compared to 50.0% for clinical perception (p = 0.015). In the SpHb group, lab hemoglobin was never less than 7 g/dL. In addition, using Bland-Altman analysis, the researchers calculated that, compared to lab hemoglobin, SpHb had bias and precision of -0.29 ± 1.03 g/dL, with limits of agreement of -2.30 and 1.72 g/dL. No difference was observed in transfusion units or postoperative hemoglobin concentrations between the two groups.
The researchers concluded, "The SpHb trend tracked changes in hemoglobin satisfactorily during surgery and more accurately estimated the appropriate timing for invasive hemoglobin measurements than the clinicians." They also noted that "This study was the first diagnostic randomized controlled trial to explore the triage role of Pulse CO-Oximetry in the intraoperative detection of anemia. We found that the trend in SpHb could detect a decrease in Hb in dynamic situations and indicate the appropriate timing for further Hb measurements."
SpHb monitoring is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Tang Bo, Yu X, Xu L, Zhu A, Zhang Y, and Huang Y. Continuous noninvasive hemoglobin monitoring estimates timing for detecting anemia better than clinicians: a randomized controlled trial. BMC Anesthesiology. 17 May 2019. https://doi.org/10.1186/s12871-019-0755-1
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of Radius PPG™, the First Tetherless SET® Pulse Oximetry Sensor Solution
Irvine, California – May 16, 2019 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance of Radius PPG, a tetherless sensor solution powered by Masimo SET® that represents a significant breakthrough in patient monitoring. Radius PPG eliminates the need for a cabled connection to a pulse oximetry monitor, allowing patients to move freely and comfortably while still being continuously monitored reliably and accurately. Via wireless connection, measurements are displayed on Masimo host devices or third-party multi-parameter monitors with integrated Masimo technology, making Radius PPG immediately available for approximately two million monitors around the world. Coupled with the proven benefits of Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, Radius PPG is suited for use anywhere patients can benefit from mobility.

Masimo Radius PPG™
Radius PPG gives patients freedom of movement without interrupting continuous monitoring. Wireless connection to a host device is simple to establish and each Radius PPG can easily pair with multiple devices (maintaining connection to any one device at a time), simplifying transfers between care areas. Radius PPG works with its integrated battery for about four days and stores up to four days (96 hours) of patient data; in the event of wireless interruption, Radius PPG provides seamless retransmission once the connection is restored. Automated by Masimo’s connectivity solutions, patient data can be used for remote clinician notifications of changes in patient condition and automatically transferred to the patient's EMR.
Studies have shown that patient mobility is a key factor in more rapid patient recovery.1,2 In addition, the removal of cables has been shown to contribute to greater patient comfort, convenience, and patient satisfaction compared to tethered patient monitoring.3 Radius PPG allows patients to move throughout the hospital room, to the bathroom, and to other care areas without the need for physical disconnection and reconnection. In places like the neonatal ICU, care providers and parents can hold infants without interrupting monitoring or risking an uncomfortable tug on the patient. Radius PPG not only offers patients improved comfort and convenience but improves clinician workflows.
Radius PPG harnesses the power of clinically proven Masimo SET® technology to provide accurate measurement even while patients move. Over 100 independent and objective studies have shown that SET® outperforms other pulse oximetry technologies during conditions of motion and low perfusion.4 When used in conjunction with Patient SafetyNet™, continuous monitoring using SET® in post-surgical wards has been shown to reduce rapid response team activations and transfers back to the ICU.5-7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity (ROP) in neonates8 and improve critical congenital heart disease (CCHD) screening in newborns.9 Today, Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.11
Joe Kiani, Founder and CEO of Masimo, said, "We are excited to announce the Radius PPG tetherless, wearable SET® pulse oximetry sensor solution. Accurate, high-quality monitoring data can now travel from an ambulating patient to a variety of monitoring platforms, allowing a patient's physiological status to be continuously monitored when it’s needed most."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Needham D et al. Archives of Physical Medicine and Rehabilitation. Vol 91, Issue 4, PP 536–542, April 2010.
2. Ronnenbaum J et al. J Acute Care Phys Ther. 2012;3(2):204-210.
3. Fensli R et al. J Med Syst (2010) 34: 767. https://doi.org/10.1007/s10916-009-9291-8.
4. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
9. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
10. Estimate: Masimo data on file.
11. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radius PPG™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radius PPG and SET®, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo and Mindray Announce Expanded Partnership
Mindray to Offer Masimo SET® Pulse Oximetry in its Patient Monitoring Devices in Additional Countries Beyond the United States
Neuchatel, Switzerland and Shenzhen, China – May 13, 2019 – Masimo (NASDAQ: MASI) and Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (SZSE: 300760) announced today that they have entered into a purchase and license agreement, under which Mindray will offer Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry – noninvasive, continuous measurement of oxygen saturation (SpO2), pulse rate (PR), and perfusion index (Pi) – in its monitoring devices. Mindray devices equipped with SET® will now be available in select countries in Europe, the Middle East, Russia and the Commonwealth of Independent States, and Asia-Pacific (excluding China), including Australia and India.
With the invention of Signal Extraction Technology® (SET®), Masimo established a new standard for pulse oximetry by introducing the ability to measure through motion and low perfusion. In a study comparing multiple pulse oximetry technologies, SET® was shown to demonstrate the highest sensitivity and specificity in identifying desaturation events and avoiding false desaturation events during these conditions.1 SET® has also opened up new frontiers in patient monitoring during challenging conditions: outcome studies have shown that SET®, combined with clinical assessment, has helped clinicians reduce retinopathy of prematurity (ROP) in neonates,2 improve critical congenital heart disease (CCHD) screening in newborns,3 and through continuous monitoring of patients in post-surgical wards, reduce ICU transfers and rapid response team activations.4-6 In all, over 100 independent and objective studies have shown that SET® outperforms other pulse oximetry technologies.7 Masimo continues to refine SET®, and recently announced that SpO2 accuracy specifications have now improved to 1.5% in conditions of motion and no motion for adult, pediatric, and infant patients (> 3 kg) with RD SET™ sensors. Now, the benefits of Masimo SET® are also available to clinicians using Mindray's devices in many countries outside the United States, where Mindray has offered SET® pulse oximetry in devices from Datascope (which began offering SET® in 1998) since acquiring Datascope in 2008.
Mindray is a leading global provider of medical devices and solutions. Mindray's products and services can be found in healthcare facilities in over 190 countries, and over 1.1 million Mindray monitors have been used or are in use across the world, representing the world's third-largest patient monitoring market share. Patient monitors now available with integrated Masimo SET® pulse oximetry include the Mindray BeneVision N and BeneView T series for use in high-acuity environments and the ePM, iPM, and iMEC series for use in a variety of clinical scenarios, among other devices.
Jon Coleman, President of Worldwide Sales, Professional Services, and Medical Affairs, Masimo, commented, "We're excited to enter into this agreement with Mindray, so that more patients, clinicians, and hospitals can benefit from the unmatched performance of Masimo SET® pulse oximetry."
Yang Ting, General Manager of International Sales and Marketing, Patient Monitoring and Life Support, Mindray, said, "We are very happy to expand our cooperation with Masimo from North America to more regions, so that our customers will have access to the outstanding SpO2 technology from Masimo. It is a proof of our constant commitment to bringing advanced medical technologies to people in need, and making better healthcare more accessible for all."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Shah N et al. Performance of Three New-Generation Pulse Oximeters During Motion and Low Perfusion in Volunteers. J Clin Anesth. 2012 Aug;24(5):385-91.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Mindray
Founded in 1991, Mindray is one of the leading global providers of medical devices and solutions. Firmly committed to our mission of "advance medical technologies to make healthcare more accessible," Mindray is dedicated to innovation in the fields of Patient Monitoring and Life Support, In-Vitro Diagnostics, and Medical Imaging System. Mindray possesses a sound global research and development, marketing and service network. Inspired by the needs of customers, Mindray adopts advanced technologies and transforms them into accessible innovation, bringing healthcare within reach. While improving the quality of care, Mindray helps reduce its cost, making it more accessible to a larger part of humanity. Today, Mindray's products and services can be found in healthcare facilities in over 190 countries and regions. In China, Mindray’s products and solutions can be found in over 110,000 medical institutions and 99% of Class A tertiary hospitals.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Vicky Wang
Mindray
Phone: 0086 755 81888775
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Reports First Quarter 2019 Financial Results
Q1 2019 Highlights
- • Total revenue, including royalty and other revenue, was $231.7 million;
- • Product revenue increased 12.8% to $230.5 million, or 14.3% on a constant currency basis;
- • Shipments of noninvasive technology boards and monitors increased 18.8% to 63,700;
- • GAAP net income per diluted share was $0.87; and
- • Non-GAAP net income per diluted share increased 23.4% to $0.79.
Irvine, California, May 6, 2019 - Masimo (Nasdaq: MASI) today announced its financial results for the first quarter ended March 30, 2019.
First Quarter 2019 Results:
Total revenue, including royalty and other revenue, was $231.7 million. Product revenue increased 12.8% to $230.5 million, or 14.3% on a constant currency basis. Shipments of noninvasive technology boards and monitors increased approximately 18.8% to 63,700 in the first quarter of 2019, compared to 53,600 in the first quarter of 2018.
GAAP operating margin was 24.2%. Non-GAAP operating margin increased 160 basis points to 24.0%, compared to 22.4% in the prior year period.
For the first quarter of 2019, GAAP net income was $49.3 million or $0.87 per diluted share. Non-GAAP net income per diluted share increased 23.4% to $0.79 per diluted share, compared to $0.64 per diluted share in the prior year period.
Total cash and short-term investments increased by $40.4 million during the quarter to reach $592.9 million as of March 30, 2019.
As a result of the strong performance in the first quarter, Masimo is raising its guidance for fiscal year 2019. The Company now expects product revenues of $918.0 million, which reflects reported growth of 10.6% and constant currency growth of 11.4%. Masimo is also raising its GAAP EPS guidance to $3.25 and its non-GAAP EPS guidance to $3.12.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “We’re off to a great start to 2019 and we are happy to report first quarter results that once again exceeded expectations. Our first quarter results illustrate the strength of our global business. Our product revenue increased 14.3% on a constant currency basis to reach $230.5 million, while we had record worldwide shipments of 63,700 noninvasive technology boards and monitors. While we are enabling more customers to improve their patient care and simultaneously helping them reduce their cost of care, our clinical leading noninvasive monitoring technologies are the driving force behind our financial success. As we celebrate our 30th anniversary, we are delighted to be able to raise our revenue and earnings guidance for 2019.”
2019 Financial Guidance
The Company provided the following updated estimates for its full year 2019 guidance:

- • Total revenue, including royalty and other revenue, increasing to $919.1 million;
- • Product revenue increasing 10.6% to $918.0 million, or 11.4% on a constant currency basis;
- • GAAP diluted earnings per share increasing to $3.25;
- • Non-GAAP diluted earnings per share increasing 17.7% to $3.12; and
- • Included in our full year revenue guidance is approximately $6.5 million of year-over-year currency headwinds.
Impact of Adoption of New Lease Accounting Standard
Effective December 30, 2018, we adopted Accounting Standards Codification (ASC) Topic 842, Leases (ASC 842). Our adoption of ASC 842 generally resulted in (a) the recognition of lessee right-of-use (ROU) assets for the right to use assets subject to operating leases; (b) the recognition of lessee lease liabilities for our obligation to make payments under operating leases; and (c) the acceleration of when we recognize certain revenue and costs as a lessor of equipment provided to end-user hospitals at no up-front charge under deferred equipment agreements with fixed multi-year sensor purchase commitments. For additional information with respect to the impact of the adoption of this new accounting standard, please reference Note 2 to our condensed consolidated financial statements that will be included in Part I, Item 1 of our Quarterly Report on Form 10-Q for the quarter ended March 30, 2019, once filed with the Securities and Exchange Commission (SEC) and Exhibit 99.3 that was included in our Current Report on Form 8-K that was filed with the SEC today.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s ongoing core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an ongoing basis: (i) constant currency product revenue growth %, (ii) non-GAAP net income, (iii) non-GAAP diluted earnings per share, (iv) non-GAAP gross profit/margin, (v) non-GAAP operating income/margin, (vi) non-GAAP product net income, (vii) non-GAAP product diluted earnings per share, (viii) non-GAAP product gross profit/margin, (ix) non-GAAP product operating income/margin and (x) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP product revenue growth percentage (%), non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency adjustments.
Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. These foreign currency revenues, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating our product revenue growth rates on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and the related constant currency adjustments for calculation of our product revenue growth rate will continue to occur in future periods.
Royalty and other revenue, net of related costs.
We derive royalty and other revenue, net of related costs, from certain non-recurring contractual arrangements that we do not expect to continue in the future. We believe the exclusion of royalty and other revenue, net of related costs, associated with these nonrecurring revenue streams is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis.
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds; most recently in connection with our November 2016 settlement agreement with Koninklijke Philips N.V. We believe that exclusion of these gains and losses is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
From time-to-time, we may experience significant non-recurring tax events, such as changes in tax laws and regulations or the derecognition of uncertain tax positions related to non-recurring transactions due to the expiration of the statutes of limitations. We believe that exclusion of such tax charges or benefits is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these tax items are unrelated to our core business and generally unique and non-recurring in nature.
First Quarter 2019 Actuals versus First Quarter 2018 Actuals




Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 2399557. After the live webcast, the call will be available on Masimo’s website through June 6, 2019. In addition, a telephonic replay of the call will be available through May 13, 2019. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 2399557.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2019 total revenue, product revenue, royalty and other revenues, gross margin, operating margin, diluted earnings per share, EBITDA, estimated tax rate and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact: Irene Paigah
Phone: (858) 859-7001
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.


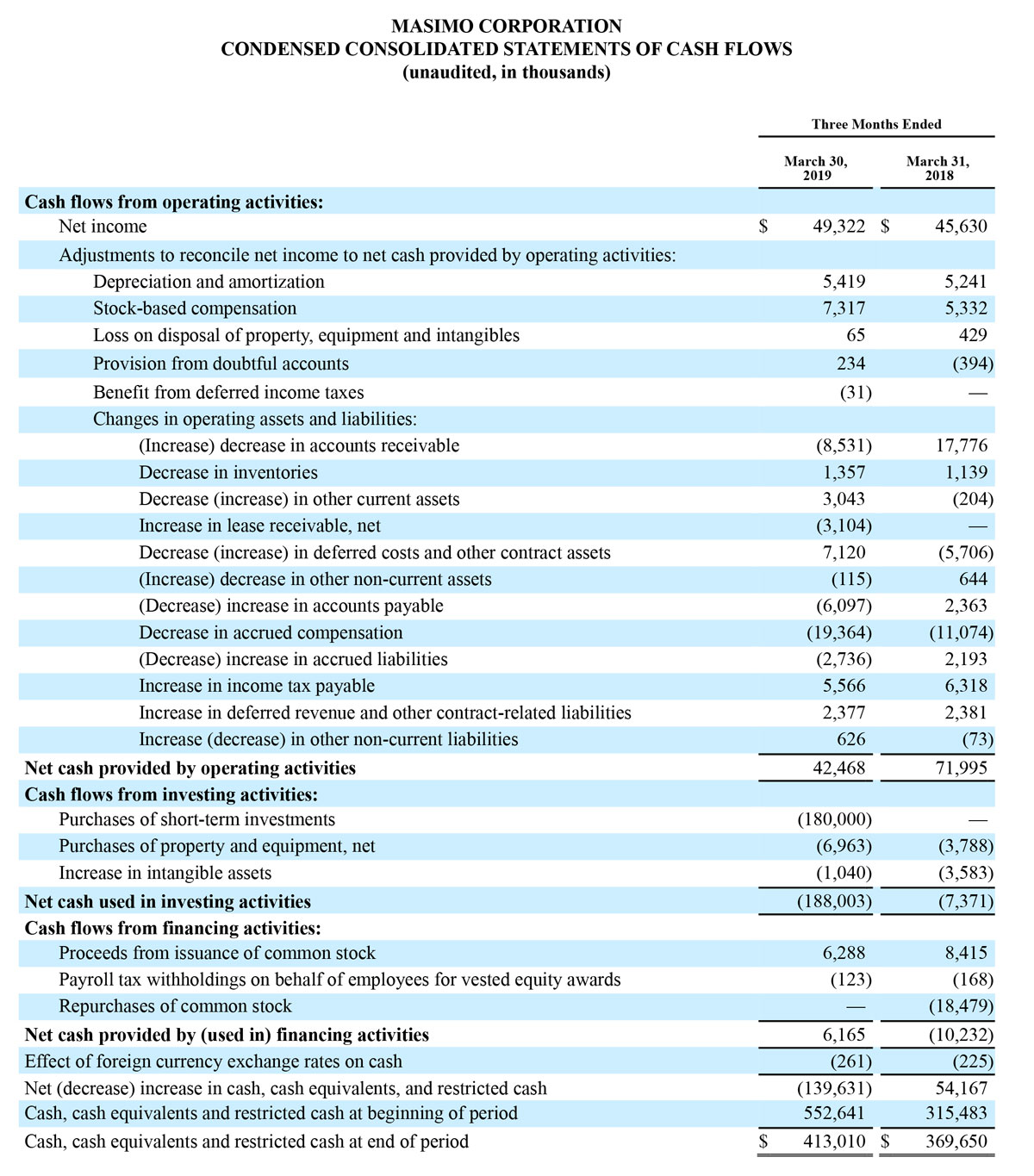

Masimo Announces Halo ION™
Irvine, California – April 30, 2019 – Masimo (NASDAQ: MASI) announced today that after a decade of developing and testing its comprehensive, scalable, and customizable continuous early warning score, with the aid of leading expert clinicians around the world, it is releasing Halo ION™. Halo ION allows clinicians to aggregate trend data from as few as three physiological parameters (oxygen saturation, pulse rate, and perfusion index), and as many as are available, including data from EMRs, into a single continuous early warning score. Each patient’s Halo ION score is displayed on the Masimo Patient SafetyNet™ Supplemental Remote Monitoring and Clinician Notification System as a number ranging from zero to 100, helping to streamline clinicians’ patient assessment workflow.
-

Masimo Patient SafetyNet™ with Halo ION™
What is difficult for people is easy for Halo ION: in calculating scores, Halo ION not only takes advantage of immediately available patient data, but more importantly, keeps track of historical physiological data and data from other records. Halo ION thus helps to automate the process by which clinicians assess patient status over time, providing a cumulative, trended score for each patient, easily visible on the Patient SafetyNet View Station or Replica™ mobile application, configured according to their clinical protocols, that can help facilitate their determination of a patient's overall status. Trends in Halo ION scores, which are calculated according to how the hospital has chosen to configure Halo ION, help clinicians evaluate whether patients are improving or deteriorating according to their own criteria. Clinicians may then use this information to, for example, intervene with certain patients, to transfer or discharge certain patients, and to schedule nursing assignment loads accordingly.
Halo ION works by continuously extracting key characteristics from clinician-selected parameters that are continuously monitored on the Root® Patient Monitoring and Connectivity Hub and anything connected to Root – such as oxygen saturation (SpO2 using Masimo SET® pulse oximetry), noninvasive hemoglobin (SpHb®), blood pressure, temperature, and pulse rate – to create an overall score. Hospitals and clinicians determine which parameters to include in the overall score, how each is configured, how each is weighted, and how combinations of changes across multiple parameters affect scoring – providing the flexibility and customizability to ensure that Halo ION reflects each institution’s assessment policy.
Unlike other early warning score assessment tools, which take spot-check snapshots of patient vital signs, Halo ION provides cumulative, continuous visibility into patient status over time, taking into account not only historical trend data for each parameter but also more complex characteristics, such as a parameter's degree of stability and variability. Halo ION creates individualized, patient-specific baseline scores for each parameter – not global, one-size-fits-all thresholds – and tracks how each patient deviates from their baselines, adjusting the overall Halo ION score differently for different patients. For example, the Halo ION score might be different for two patients whose oxygen saturation drops to 89% if one patient normally has 100% SpO2 and the other 94%; those different deltas would be reflected in different amounts of change in their Halo ION scores.
Joe Kiani, Founder and CEO of Masimo, commented, "Precision medicine is no longer only about the right drug for the right patient based on their genetics, but also about individualized assessment of their state of health. Halo ION represents many years of research into how best to take the complex, almost overwhelming stream of high quality physiological data available to clinicians and present it in a way that is intuitive, efficient, and provides individualized insight into a patient's overall status. With its unique ability to create patient-specific baselines and to take into account many subtle changes over time, Halo ION helps automate and simplify the process of patient assessment, helping clinicians stay focused on providing the best care for their patients."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET™ sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97™. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway™, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Estimate: Masimo data on file.
8. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Halo ION™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Halo ION, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces First CE-marked Third-party Masimo Open Connect® Module for the Root® Patient Monitoring and Connectivity Hub
Neuchatel, Switzerland – April 29, 2019 – Masimo (NASDAQ: MASI) and Mdoloris Medical Systems announced today the CE marking of the Mdoloris Analgesia Nociception Index (ANI®) module for the Masimo Root® Patient Monitoring and Connectivity Hub, the first commercially available result of a Masimo Open Connect® (MOC) third-party partnership, between Mdoloris and Masimo.
-

Masimo Root® with Next Generation SedLine® Brain Function Monitoring and Mdoloris ANI®
Masimo's unique approach to medical technology integration through Masimo Open Connect partnerships addresses some of the major barriers to new technology adoption in patient monitoring. The Root platform’s open architecture and advanced connectivity enable third-party companies to bypass barriers and the time it takes for traditional multi-parameter monitor integration by controlling their own Root integration projects. Third parties can then independently develop, obtain regulatory approvals, and commercialize their own external MOC-9® module or MOC-C® app for Root using Masimo's MOC software development kit and support from Masimo’s engineering and distribution teams.
Joe Kiani, Founder and CEO of Masimo, said, "We are proud to announce ANI, the first commercially available third-party MOC-9 module for Root, the first of many to come. With the ongoing expansion of its capabilities, Root becomes a more powerful bedside platform than ever. We believe that Root with Masimo Open Connect can do for patient monitoring what the PC did for computing: speed up the patient monitoring innovation cycle, reduce the cost of patient monitoring, and prolong the useful life of the equipment hospitals invest in."
"We are delighted to be able to announce that ANI is now cleared for sale in the EU," said Fabien Pagniez, Founder and CEO of Mdoloris Medical Systems. "We consider Masimo to be the most innovative company in the patient monitoring space and we believe Root offers a unique and compelling solution for implementing our ANI technology."
ANI on Masimo Root has not received 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.8 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Estimate: Masimo data on file.
8. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Mdoloris Medical Systems
Mdoloris (www.mdoloris.com), a medical devices manufacturer, was created in June 2010 out of 23 years of academic research performed in Lille University hospital, France. It has an international representation in more than 64 countries and a scientific, technical and medical acknowledgement. Mdoloris has so far developed three products, all able to continuously assess the pain level of patients (the ANI technology for patients older than two years old, the NIPE technology for neonates, and the PTA technology for pets). Its innovative technologies provide clinical added value for clinicians who are not able to communicate with their patients in order to personalize pain medications and avoid known side effects due to over- and under-dosage of such drugs. More than 1,200 devices are now used by anesthesiologists and intensivists worldwide and more than 140,000 patients have benefited from Mdoloris’ technologies. Mdoloris’s standalone monitor promoting its innovative adult technology has received 510(k) clearance through the HFVI brand in the US under the number K142969. Mdoloris recently closed a nine-million euro fundraising round in order to keep developing new-to-the-world technologies that always keep one target in mind: helping clinicians improve the quality of care.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Pierrick Niewiadowski
Mdoloris
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo to Report First Quarter 2019 Financial Results after Market Close on Monday, May 6
IRVINE, Calif – April 15, 2019 – Masimo (NASDAQ: MASI) announced today that it will release first quarter 2019 financial results for the period ended March 30, 2019, after the market closes on Monday, May 6, 2019. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Micah Young, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 2399557. After the live webcast, the call will be available on Masimo's website through June 6, 2019. In addition, a telephonic replay of the call will be available through May 13, 2019. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 2399557.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
Investor Contact: Eli Kammerman
Phone:(949) 297-7077
Email: [email protected]
Media Contact: Irene Paigah
Phone:(858) 859-7001
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Investigates Cerebral Oxygenation Normative Values Using Masimo O3® Regional Oximetry
Neuchatel, Switzerland – April 15, 2019 – Masimo (NASDAQ: MASI) announced today the findings of a study, recently published in Anaesthesia and Intensive Care, in which researchers at Austin Hospital in Melbourne, Australia sought to measure cerebral oxygenation in a large cohort of healthy volunteers, using Masimo O3® Regional Oximetry, to establish a normal range of values and investigate the relationship between cerebral oxygenation and other physical and hemodynamic characteristics.1 O3 uses near-infrared spectroscopy (NIRS) to monitor cerebral oxygenation in situations in which peripheral pulse oximetry alone may not be fully indicative of the oxygen in the brain.
-

Masimo Root® with O3® Regional Oximetry and Next Generation SedLine® Brain Function Monitoring
Noting that "normative values for clinical devices are essential to allow for definition of abnormality during clinical use" and that available regional cerebral tissue oxygen saturation (SctO2) reference values have limitations, including not taking into account variables such as brain hemisphere, sex, skin type, height, weight, and others, Dr. Christopher Eyeington and colleagues used Masimo O3 to assess for differences in SctO2 between hemispheres, sex, and comorbidity and smoking status, and for associations between SctO2 and key physical and hemodynamic characteristics, in healthy adults. They enrolled 98 volunteers, 22-60 years old, including 41 males, 22 with one or more co-morbidities, 13 current or former smokers, and with a variety of skin types. Each volunteer was monitored continuously for five minutes using O3 on the Masimo Root® Patient Monitoring and Connectivity Hub, with SctO2 measurements recorded every two seconds.
The researchers recorded 32,130 SctO2 observations. Mean left, right, and combined average SctO2 values were 67.3%, 67.9%, and 67.6%, respectively, with a "narrow" combined average 95% confidence interval of 66.8% to 68.6%. (None of the 95% confidence intervals was lower than 66.5% or greater than 69.1%.) The researchers found "statistically significant yet quantitatively small differences" in SctO2 values according to hemisphere (p < 0.001). They also found that increasing mean arterial pressure (MAP) (p = 0.001) and cardiac index (CI) (p ≤ 0.001) were associated with increased SctO2: each 10 mmHg increase in MAP and 1 L/min/m2 increase in CI was associated with 0.01% and 0.1% increases in SctO2, respectively.
The researchers noted, "Our study implies that in healthy adults the mean SctO2 measured with modern technology is close to 68% with narrow confidence intervals of 1%, and with no difference between hemispheres. Moreover, given a lowest mean combined bi-hemispheric SctO2 value of 56%, it implies that an SctO2 value below 56% should be considered 'abnormally low.' In addition, the very few SctO2 values below 60% seen in our study imply that persistent measurements below such a threshold should be viewed with concern. Finally, our observation that SctO2 values were not affected in any clinically significant amount by hemispheres, sex, skin type, comorbidity or smoking status, age or any haemodynamic parameter implies that in healthy adults, cerebral tissue oxygen saturation is unaffected by these factors." They concluded that "These findings have significant implications regarding the clinical interpretation of SctO2 and the application of this information to individual patients."
Study co-author Dr. Rinaldo Bellomo commented, "The estimation of cerebral oxygenation by near infrared spectroscopy (NIRS) during anesthesia or in critical illness is becoming increasingly recognized as a desirable form of monitoring. Thus, it is vital for clinicians to understand normal values and to have confidence in the technology behind such measurements. The recent study by my colleagues and myself used >30,000 observations in close to 100 normal subjects and found that the mean normal value for cerebral oxygen estimation using modern Masimo NIRS technology was 67.6%, and, more importantly, that the 95% confidence interval for such value was narrow, between 66.8% and 68.6%. Such findings provide a reference value for patient assessment and give a degree of confidence to clinicians in relation to the validity and robustness of this technology."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Eyeington C, Ancona P, Osawa E, Cutuli S, Eastewood G and Bellomo R. Modern technology-derived normative values for cerebral tissue oxygen saturation in adults. Anaesthesia and Intensive are. 2019. DOI: 10.117.7/0310057X18811962.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of the Rad-67™ Pulse CO-Oximeter® with Next Generation SpHb® Spot-check Monitoring & rainbow® DCI®-mini Reusable Sensor
Irvine, California – March 25, 2019 – Masimo (NASDAQ: MASI) announced today FDA clearance of the Rad-67™ Pulse CO-Oximeter® with Spot-check Next Generation SpHb® monitoring technology and the rainbow® DCI®-mini Reusable Sensor. Rad-67 offers rainbow® noninvasive hemoglobin measurement (SpHb) and Measure-through Motion and Low Perfusion™ SET® pulse oximetry in a compact, portable spot-check monitoring device. When used with the rainbow® DCI-mini sensor, Rad-67 provides spot-check monitoring with Next Generation SpHb.

Masimo Rad-67™
Next Generation SpHb technology significantly advances the forefront of noninvasive portable hemoglobin spot-check monitoring: SpHb field performance is enhanced across all hemoglobin ranges through faster measurement results and improved repeatability.1
Rad-67's ability to provide portable spot-check monitoring measurements of both oxygen saturation and noninvasive hemoglobin makes it a single-device solution in multiple clinical and non-clinical settings, such as emergency rooms, pre-/post-surgery settings, and physicians' offices. Rad-67 features a rechargeable battery with six-hour run time, powering a high-resolution color display with intuitive touchscreen navigation that automatically adjusts brightness to optimize visibility in a variety of care settings. Rad-67's feedback screens provide real-time user guidance during SpHb measurement to help reduce error, while the slim-profile sensor connector port is designed to provide tactile feedback upon proper connection.
Rad-67 provides convenient historical data review directly on the device, with unique patient identifiers to help improve organization of records and workflow. Built-in wireless connectivity will enable data transfer to the EMR and printing results at the point of care, which may help reduce the likelihood of transcription errors2 or incomplete case records.
Professor Andrew Klein, Chair of the Department of Anesthesia and Intensive Care at Royal Papworth Hospital in Cambridge, England, and Editor-in-Chief of Anaesthesia, commented, "The Rad-67 is incredibly easy to use and gives a quick result, perfect in our busy pre-operative clinic. We have found it particularly useful as it helps us target those patients who may need further testing."
Joe Kiani, Founder and CEO of Masimo, said, "We're proud to launch Rad-67, Spot-check Next Generation SpHb monitoring, and the rainbow® DCI-mini in the United States. Spot-check Next Generation SpHb monitoring represents a significant enhancement to the noninvasive measurement we invented a decade ago – a measurement we look forward to continuing to improve."
Rad-67 spot-check SpHb monitoring is not cleared for use on pediatric patients, pregnant patients, and patients with renal disease. SpHb is not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision-making.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.6-8 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.10 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Masimo field data on file.
2. The Value of Medical Device Interoperability. West Health Institute. 2013.
3. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
4. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
5. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
6. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
7. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
8. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
9. Estimate: Masimo data on file.
10. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-67™, rainbow® DCI®-mini sensor, and Next Generation SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-67, rainbow® DCI-mini sensor, and Next Generation SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

St. Luke’s University Health Network Achieves Compelling Outcome Results Following Implementation of Masimo Supplemental Remote Monitoring Solution
Non-profit Pennsylvania Network Is Expanding Use of Masimo Patient SafetyNet™, Root® with Radius-7® and Vital Signs Check to Almost 500 Beds Across 10 Hospitals
Bethlehem, Pennsylvania – March 19, 2019 – St. Luke's University Health Network (St. Luke's) and Masimo (NASDAQ: MASI) announced today that St. Luke's, a regional network of 10 hospitals and 320 affiliated sites providing service to 10 counties in eastern Pennsylvania, is expanding their use of a variety of Masimo technologies following impressive outcome results at a pilot site.
Four years ago, seeking to improve patient safety and reduce morbidity and mortality in their hospitals, St. Luke's formed a multidisciplinary taskforce – comprised of anesthesiologists, nurses, respiratory specialists, hospital leaders, and others – charged with implementing changes and tracking outcomes in a pilot location, a 34-bed orthopedic trauma ward at their hospital in Bethlehem. As part of that program, which also involved changes in clinical practice and alarm management, St. Luke's installed Masimo Patient SafetyNet™, a supplemental remote monitoring and clinician notification system, in the pilot ward. Patients were monitored at the bedside using the Masimo Root® patient monitoring and connectivity hub in conjunction with Masimo Radius-7® tetherless, wearable Pulse CO-Oximeters® that continuously monitor patients' oxygen saturation and pulse rate using Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry. Through Patient SafetyNet, these and other parameters can be continuously remotely monitored from central view stations and even smartphones, with the ability to alert clinicians from afar to possible deterioration in patient condition. In 2016, a year after implementation of the program, clinicians achieved impressive outcome and financial results compared to 2015 performance, including a 62% reduction in mortality, a 36% reduction in naloxone administration, a 23% reduction in the utilization of telemetry, a 26% reduction in critical care transfers, and an estimated savings of $900,000 in cost avoidance.
"Because of the unpredictability of which patients will deteriorate, continuous and in-depth monitoring provides a valuable level of data that can be acted upon quickly to save lives," said Aldo Carmona, MD, Chairman of the Department of Anesthesia and Critical Care and SVP of Clinical Integration, who leads the initiative. "Constant monitoring of changes in patient conditions will alert doctors and nurses when gradual deterioration is sensed, enabling a quicker therapeutic response and avoiding emergent situations."
Following the successful pilot program, St. Luke's expanded use of Patient SafetyNet to an additional 48 beds across two additional units on their Bethlehem campus. Now, three years later, the network is again expanding use of Patient SafetyNet and other Masimo solutions to almost 500 beds in total, so that all eight existing hospitals will have the technology – and in two hospitals slated to open this year, only Masimo continuous monitoring technologies will be used outside the ICU. Monitoring data is now automatically transferred from bedside devices and Patient SafetyNet to St. Luke’s Epic electronic medical record (EMR) system, helping improve productivity and reducing the likelihood of transcription errors.1 Vital Signs Check, an application for Root designed to streamline vital signs measurement workflows and optimize patient data management, is also being implemented. "With the higher acuity levels of many hospitalized patients, taking vital signs over four or eight hours is no longer effective in many cases, and developing conditions are missed until they become critical threats sometimes resulting in poor outcomes," Dr. Carmona said.
Speaking of the ongoing expansion and enhancement of their continuous monitoring program, Dr. Carmona commented, "This Patient SafetyNet initiative will be the most important patient safety project I will work on in my whole career."
St. Luke's sought inspiration from another institution's successful implementation of continuous monitoring using Masimo SET® and Patient SafetyNet: Dartmouth-Hitchcock Medical Center in New Hampshire, where researchers found that continuous monitoring of adult post-surgical patients using Masimo SET® pulse oximetry on Masimo bedside devices, in conjunction with Masimo Patient SafetyNet, resulted in a 65% reduction in rapid response team activations and a 48% reduction in transfers back to the ICU.2 Over five years, Dartmouth-Hitchcock achieved their goal of zero preventable deaths or brain damage due to opioids,3 and over ten years, they maintained a 50% reduction in unplanned transfers and a 60% reduction in rescue events, despite increases in patient acuity and occupancy.4 With the monitoring of additional physiological parameters and integration into EMRs, St. Luke’s initiative is set to provide even more continuous supplemental monitoring coverage than at Dartmouth-Hitchcock.
Joe Kiani, Founder and CEO of Masimo, said, "We are honored to partner with St. Luke's as they continue to expand their patient safety and monitoring initiatives. Our mission – to improve patient outcomes and reduce the cost of care – aligns well with their values. Their ongoing commitment to saving lives through our proven SET® and Patient SafetyNet technology is a model we hope more and more institutions will see the benefits of implementing."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,6 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.2-4 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. The Value of Medical Device Interoperability. West Health Institute. 2013.
2. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
3. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
4. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
5. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
6. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
7. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About St. Luke's
Founded in 1872, St. Luke's University Health Network (SLUHN) is a fully integrated, regional, non-profit network of more than 15,000 employees providing services at 10 hospitals and over 320 outpatient sites. With annual net revenue greater than $2 billion, the Network’s service area includes 10 counties: Lehigh, Northampton, Berks, Bucks, Carbon, Montgomery, Monroe and Schuylkill counties in Pennsylvania and Warren and Hunterdon counties in New Jersey. Dedicated to advancing medical education, St. Luke's is the preeminent teaching hospital in central-eastern Pennsylvania. In partnership with Temple University, St. Luke's created the Lehigh Valley's first and only regional medical school campus. It also operates the nation’s longest continuously operating School of Nursing, established in 1884, and 28 fully accredited graduate medical educational programs with 226 residents and fellows. St. Luke's is the only Lehigh Valley-based health care system with Medicare’s five- and four-star ratings (the highest) for quality, efficiency and patient satisfaction. St. Luke's is both a Leapfrog Group and Healthgrades Top Hospital. In 2019, three of IBM Watson Health’s 100 Top Hospitals were St. Luke's hospitals. St. Luke’s University Hospital has earned the 100 Top Major Teaching Hospital designation from IBM Watson Health seven times total and five years in a row. St. Luke’s has also been cited by IBM Watson Health as a 50 Top Cardiovascular Program. Utilizing the Epic electronic medical record (EMR) system for both inpatient and outpatient services, the Network is a multi-year recipient of the Most Wired award recognizing the breadth of the SLUHN's information technology applications such as telehealth, online scheduling and online pricing information. St. Luke's is also recognized as one of the state's lowest cost providers.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™, SET®, Root®, and Radius-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, SET®, Root, and Radius-7, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Sam Kennedy, Corporate Communications Director
St. Luke's
Phone: (484) 526-4134
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces CE Marking of Pediatric Indication for Next Generation SedLine® Brain Function Monitoring
Next Generation SedLine Now Available for Use on Patients 1-18 Years Old in CE Mark Countries
Neuchatel, Switzerland – March 4, 2019 – Masimo (NASDAQ: MASI) announced today the CE marking of Next Generation SedLine® brain function monitoring for pediatric patients (1-18 years of age). With this clearance, the benefits of Next Generation SedLine are available for all patients one-year-old and above in CE mark countries. SedLine helps clinicians monitor the state of the brain under anesthesia with bilateral data acquisition and processing of four leads of electroencephalogram (EEG) signals.
-

Masimo Root® with Next Generation SedLine®
SedLine uses a pediatric-specific signal processing engine to improve performance of Masimo’s processed EEG parameter, the Patient State Index (PSi), when monitoring pediatric patients one-year-old and above. The use and monitoring of anesthesia on pediatric patients can differ from its use on adults.1-2 Maintaining an appropriate level of anesthesia is key to preventing anesthesia-related events and enabling faster recovery.3 The PSi in SedLine, driven by its pediatric-specific engine, is uniquely suited to helping clinicians interpret the EEGs of this challenging population.
In addition to the newly announced pediatric-specific signal processing when used on pediatric patients, Next Generation SedLine offers significant improvements over original SedLine, including:
- • A PSi with less susceptibility to electromyography (EMG) interference.
- • A Multitaper Density Spectral Array (DSA), which may enhance visibility of EEG features.
Joe Kiani, Founder and CEO of Masimo, said, "Next Generation SedLine is doing for brain function monitoring what Masimo SET® did for pulse oximetry. We believe Next Generation SedLine is the best and most advanced way to monitor depth of sedation, crucial to helping ensure patients with even the most challenging brains are appropriately anesthetized. We are gratified that its benefits are now available to those younger patients whose brains are particularly delicate and to whose wellbeing Masimo has always been so committed."
Next Generation SedLine has received FDA clearance for adults but is not currently indicated for pediatric patients in the USA.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.7-9 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.11 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Davidson et. Current Anesthesiology Reports 3. 1 (2013): 57-63.
2. Cornelissen L et al. Elife 4 (2015): e06513.
3. Musialowicz et al. Current Anesthesiology Reports 4. 3 (2014): 251-260.
4. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
5. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
6. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
7. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
8. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
9. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
10. Estimate: Masimo data on file.
11. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine® and Next Generation SedLine. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine and Next Generation SedLine, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Reports Fourth Quarter and Full Year 2018 Financial Results
Fourth Quarter 2018 Highlights (compared to Fourth Quarter 2017):
- • Total revenue, including royalty and other revenue, was $223.1 million;
- • Product revenue increased 12.8% to $221.4 million, or 13.5% on a constant currency basis;
- • Shipments of noninvasive technology boards and monitors increased 11.5% to 60,300;
- • GAAP net income per diluted share of $0.83; and
- • Non-GAAP net income per diluted share increased 53.7% to $0.83.
Full Year 2018 Highlights (compared to Full Year 2017):
- • Total revenue, including royalty and other revenue, was $858.3 million;
- • Product revenue increased 12.4% to $829.9 million, or 11.9% on a constant currency basis;
- • Shipments of noninvasive technology boards and monitors increased 14.1% to 231,700;
- • GAAP net income per diluted share of $3.45; and
- • Non-GAAP net income per diluted share increased 31.7% to $3.03.
Irvine, California, February 26, 2019 - Masimo (Nasdaq: MASI) today announced its financial results for the fourth quarter and full year ended December 29, 2018.
Fourth Quarter 2018 Results (compared to Fourth Quarter 2017):
Total revenue, including royalty and other revenue was $223.1 million. Product revenues increased 12.8% to $221.4 million, or 13.5% on a constant currency basis. The company shipped approximately 60,300 noninvasive technology boards and monitors.
GAAP operating margin was 24.2%. Non-GAAP operating margin increased 260 basis points to 24.3%, compared to 21.7%. Excluding the impact of royalty and other revenue, non-GAAP product operating margin increased 640 basis points to 23.8%, compared to 17.4% in the prior year period.
GAAP net income per diluted share was $0.83. Non-GAAP net income per diluted share increased 53.7% to $0.83, compared to $0.54 in the prior year period. Excluding the impact of royalty and other revenue, non-GAAP product net income per diluted share increased 97.6% to $0.81, compared to $0.41 in the prior year period.
Full Year 2018 Results (compared to Full Year 2017):
Total revenue, including royalty and other revenue was $858.3 million. Product revenue increased 12.4% to $829.9 million, or 11.9% on a constant currency basis. The Company shipped approximately 231,700 noninvasive technology boards and monitors.
GAAP operating margin was 24.2%. Non-GAAP operating margin increased 100 basis points to 24.5%, compared to 23.5% in the prior year period. Excluding the impact of royalty and other revenue, non-GAAP product operating margin increased 340 basis points to 22.0%, compared to 18.6% in the prior year period.
GAAP net income per diluted share was $3.45. Non-GAAP net income per diluted share increased 31.7% to $3.03, compared to $2.30 in the prior year period. Excluding the impact of royalty and other revenue, non-GAAP product net income per diluted share increased 53.2% to $2.65, compared to $1.73 in the prior year period.
Total cash and cash equivalents increased $237.2 million during the year to $552.5 million as of December 29, 2018.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “2018 was a dynamic year for Masimo as we are seeing strong momentum in our business. Our global organization executed on our strategy to deliver above-market growth and drive operational efficiencies throughout the business. We are extremely happy to report fourth quarter and full year results that exceeded expectations. Due to the strong finish in 2018, we are now increasing our 2019 product revenue guidance to $912 million and our 2019 non-GAAP earnings per diluted share guidance to $3.08.”
2019 Financial Guidance:
The Company provided the following estimates for its full year 2019 guidance:

- • Total revenue, including other revenue, of $912 million;
- • Product revenue of $912 million, which reflects growth of 9.9% and constant currency growth of 10.7%;
- • Non-GAAP product operating margin of 24.0%, increasing 200 basis points over prior year period;
- • Non-GAAP product earnings per diluted share of $3.08, increasing 16.2% over the prior year period; and
- • Included in our full year revenue guidance is approximately $7.0 million of year-over-year currency headwinds.
Impact of Adoption of New Revenue Accounting Standard:
During the first quarter of 2018, the Company adopted Financial Accounting Standards Board (FASB) Accounting Standards Update (ASU) No. 2014-09, Revenue (Topic 606): Revenue from Contracts with Customers (ASU 2014-09). The Company’s adoption of ASU 2014-09 generally resulted in (a) the acceleration of when the Company recognizes certain revenue, and (b) the deferral of certain incremental costs associated with obtaining a customer contract. In accordance with the full retrospective method of adoption for ASU 2014-09, the Company has adjusted certain amounts previously reported in its condensed consolidated financial statements to comply with the new standard, as indicated by the notation, “As Adjusted”. For additional information with respect to the impact of the adoption of this new accounting standard and reconciliations to the prior reported amounts, please reference Note 2 to our consolidated financial statements that will be included in Part IV, Item 15(a)(1) and 15(a)(2), respectively, of our Annual Report on Form 10-K for the fiscal period ended December 29, 2018 that will be filed with the Securities and Exchange Commission on or about February 26, 2019.
Impact of Adoption of New Lease Accounting Standard:
In February 2016, the Financial Accounting Standards Board issued a new standard for leases, Accounting Standards Codification (ASC) Topic 842, Leases (ASC 842). This standard will become effective for the Company on December 30, 2018. The Company is continuing to evaluate the expected impact of ASC 842 on its consolidated financial statements, but anticipates that, among other things, the required recognition by a lessee of a lease liability and related right-of-use asset for operating leases will increase both the assets and liabilities recognized and reported on its balance sheet as of the adoption date. In addition, ASC 842 will also change the classification of certain leases for which the Company is the lessor, resulting in the acceleration of revenue under certain contracts, as well as the immediate expensing of certain costs that are currently deferred and expensed over the life of the lease. The Company is continuing to evaluate the available practical expedients and its adoption method for this new standard.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s ongoing core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an ongoing basis: (i) constant currency product revenue growth %, (ii) non-GAAP net income, (iii) non-GAAP diluted earnings per share, (iv) non-GAAP gross profit/margin, (v) non-GAAP operating income/margin, (vi) non-GAAP product net income, (vii) non-GAAP product diluted earnings per share, (viii) non-GAAP product gross profit/margin, (ix) non-GAAP product operating income/margin and (x) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP product revenue growth %, non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency adjustments.
Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. These foreign currency revenues, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating our product revenue growth rates on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and the related constant currency adjustments for calculation of our product revenue growth rate will continue to occur in future periods.
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds. We believe that exclusion of these gains and losses is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
From time to time, we record tax benefits relating to the derecognition of uncertain tax positions due to the expiration of the statutes of limitations. We believe that exclusion of the tax benefit resulting from the expiration of certain statutes of limitations related to non-recurring transactions is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that this tax item is unrelated to our core business and non-recurring in nature.
Fourth Quarter and Full Year 2018 Actuals versus Fourth Quarter 2017 and Full Year Actuals:



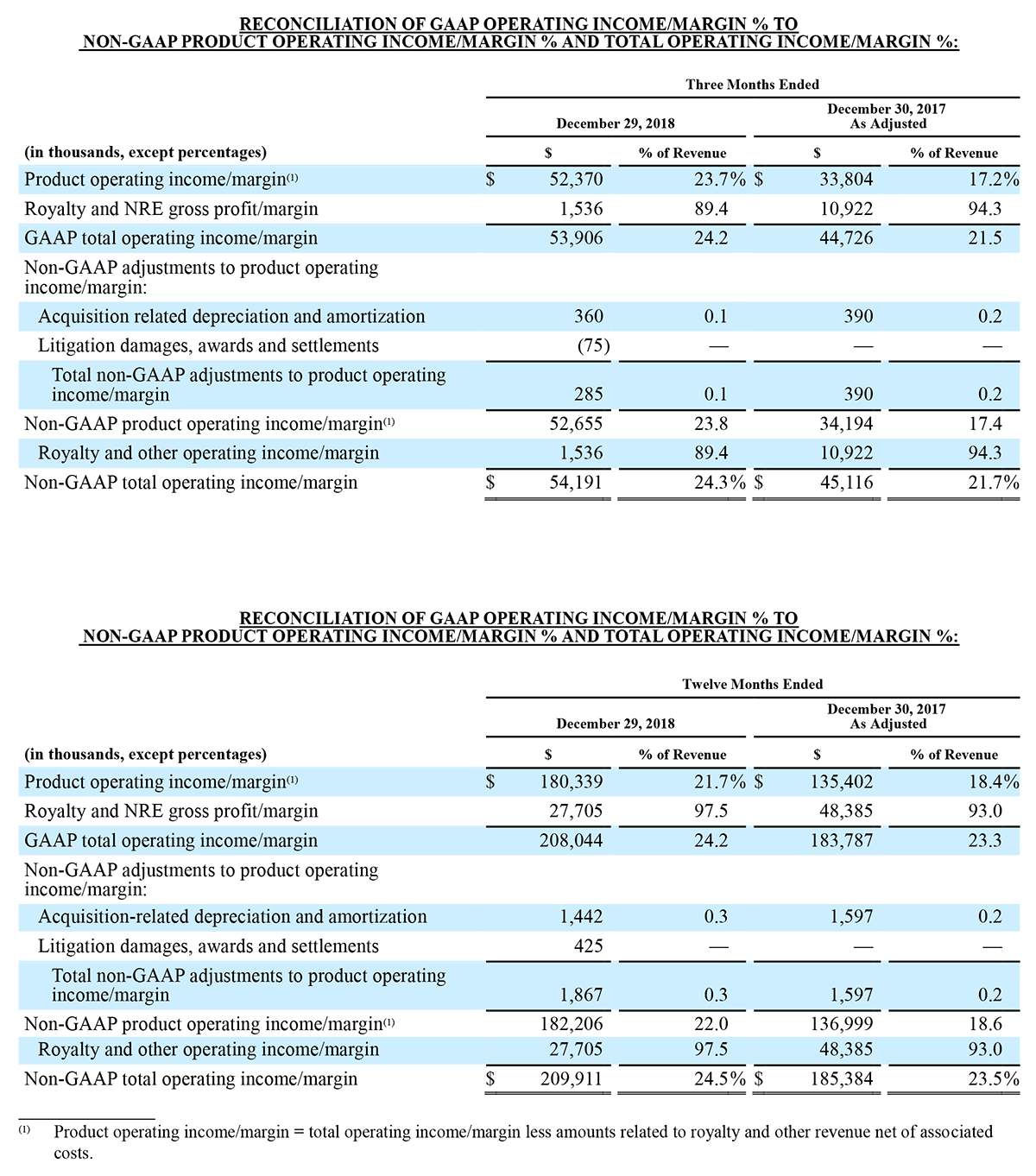
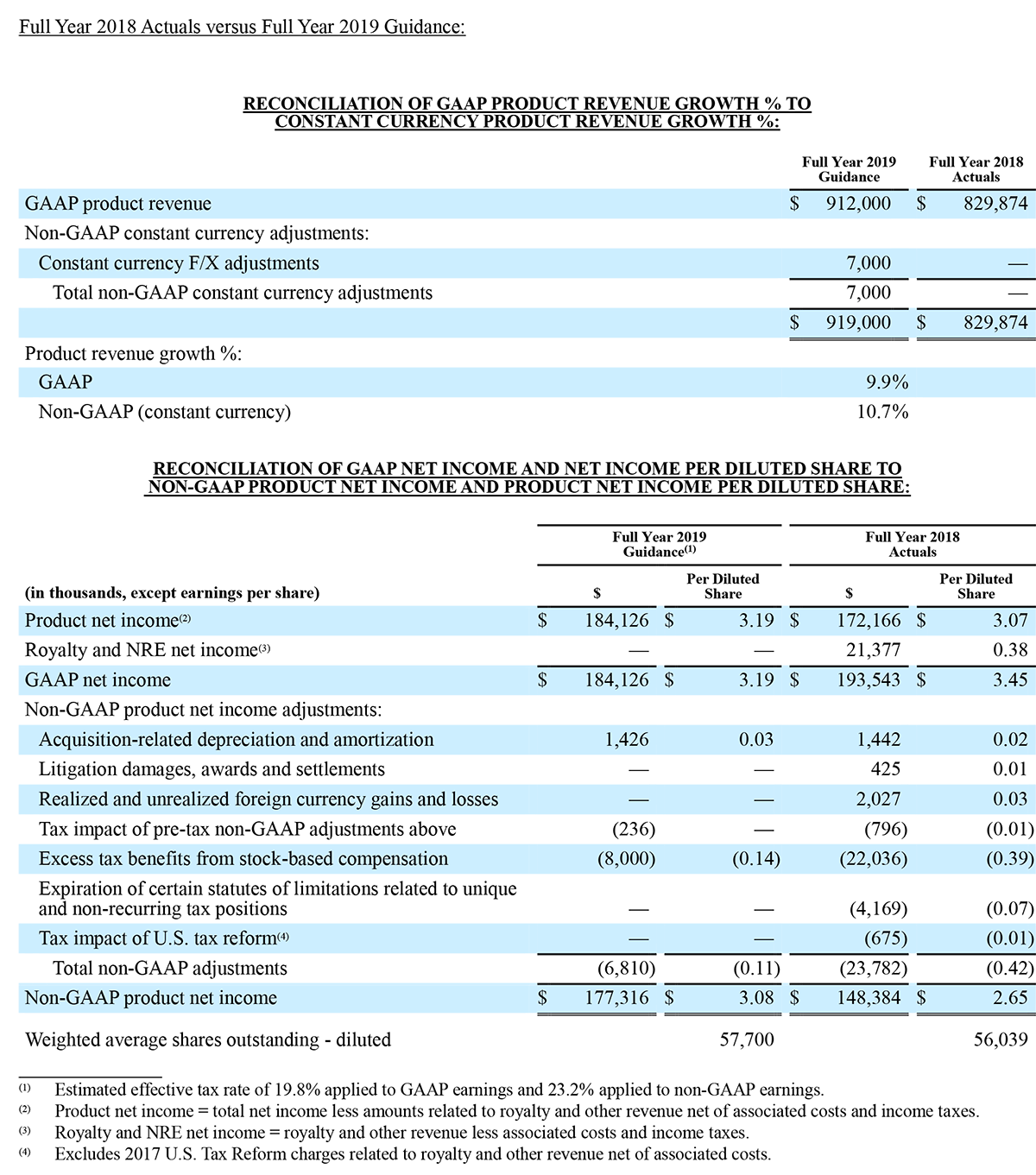
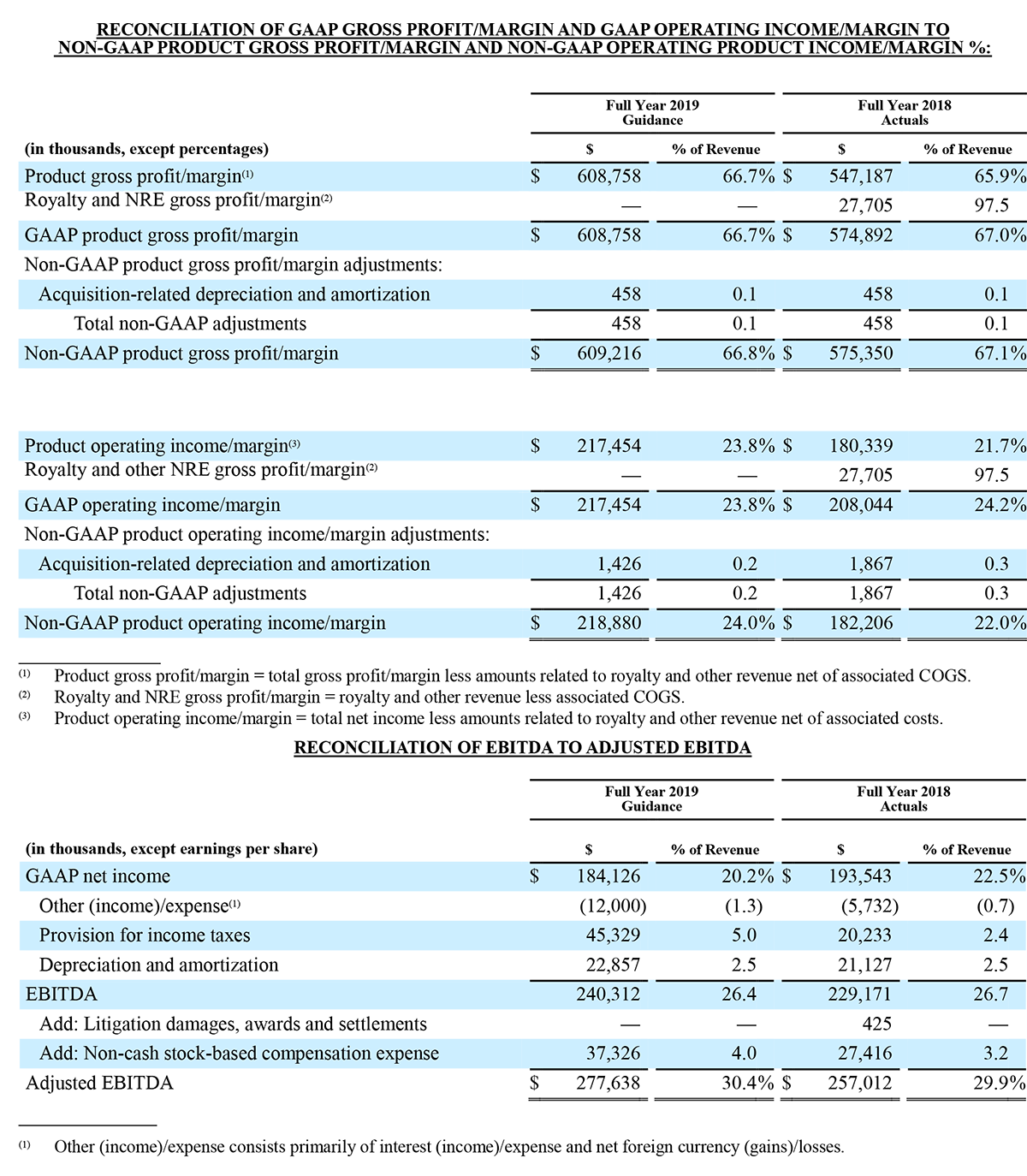
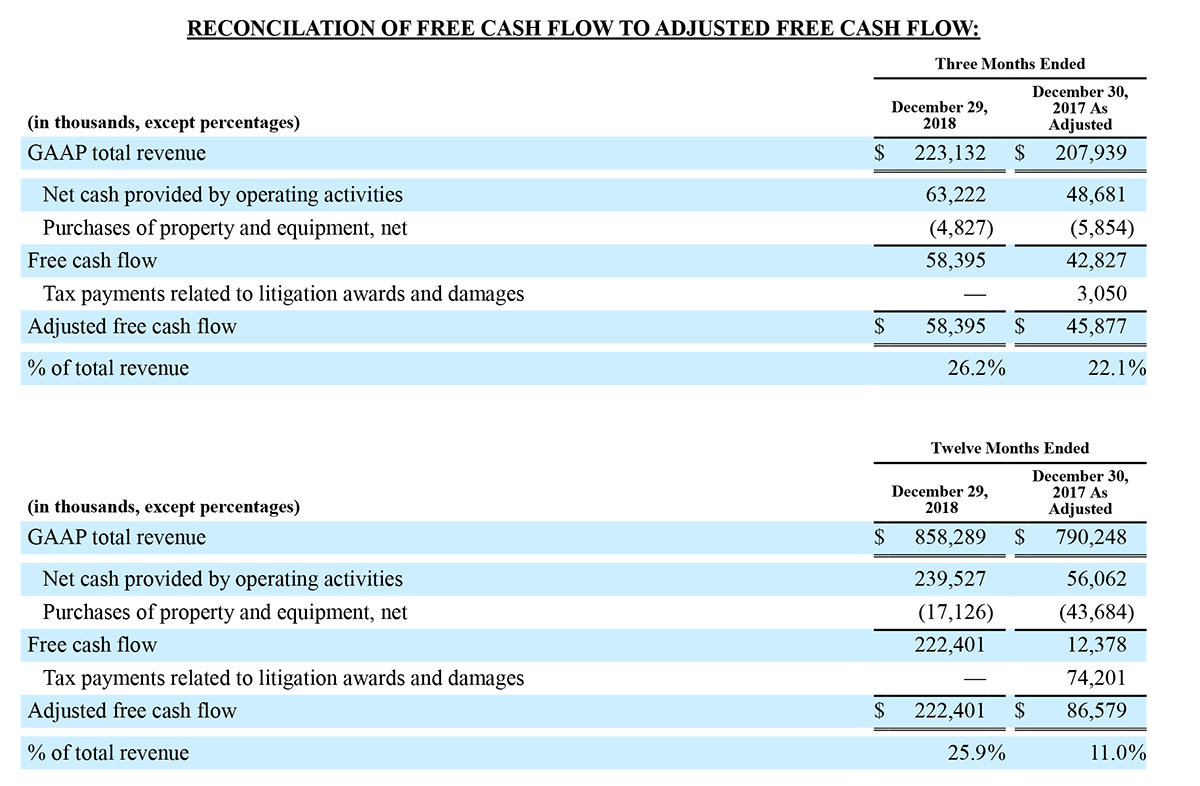
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 6889465. After the live webcast, the call will be available on Masimo’s website through March 27, 2019. In addition, a telephonic replay of the call will be available through March 6, 2019. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 6889465.
About Masimo
Masimo (Nasdaq: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat® fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2019 total, product, royalty and other revenues, gross profit/margin, earnings per diluted share, product earnings per diluted share, operating margin, product operating income/margin, net income, product net income, EBITDA, adjusted EBITDA , free cash flow, and estimated tax rate, and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact: Irene Paigah
Phone: (858) 859-7001
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.

New Case Series Investigates the Combined Use of Masimo SedLine® Brain Function Monitoring and O3® Regional Oximetry During Cardiac Surgery
Neuchatel, Switzerland – February 25, 2019 – Masimo (NASDAQ: MASI) announced today the findings of a series of four clinical cases, recently published in the Canadian Journal of Anesthesiology, in which researchers at the University of Montreal found that combined use of Masimo SedLine® brain function monitoring and O3® regional oximetry assisted their understanding and management of cerebral desaturations during cardiac surgery.1
-

Masimo Root® with SedLine® and O3®
Drs. Etienne Couture, Alain Deschamps, and André Denault hypothesized that the addition of processed electroencephalography (pEEG) using Masimo SedLine’s processed EEG parameter, the Patient State Index (PSi), to a previously developed clinical management algorithm based on Masimo O3 near-infrared spectroscopy (NIRS), could help guide the management of cerebral desaturation episodes. In this series of case studies, they describe the impact of combining the modalities on the clinical management of four patients undergoing cardiac surgery. The researchers then outline a series of scenarios that enumerate possible causes of desaturation based on various combinations of changes in NIRS and pEEG, as well as changes in related monitoring data available via SedLine, such as the density spectral array (DSA) and spectral edge frequency (SEF). Possible causes include a change in cerebral blood flow, cerebral hypoperfusion, cardiogenic shock, hypoxemia, a change in the anesthetic state, hyperthermia, and seizure. In the four cases, more insight into the likely cause of a desaturation episode helped guide how clinicians responded during surgery.
The researchers concluded, "Combining both NIRS and pEEG allows for a much more nuanced understanding of the etiology of cerebral desaturation. Future studies are needed to investigate if the combination of both modalities is more prognostic than each alone. Every cerebral oxygen desaturation is not equal."
Dr. Denault commented, "By combining NIRS and pEEG we were able to have a better appreciation of the significance of brain desaturation, and using Masimo O3 and SedLine in particular to obtain those measurements provided a number of advantages. The additional brain monitoring parameters available through Masimo SedLine, such as the DSA and SEF, play an important role in our protocol, helping to provide additional insight. And, perhaps most significantly, both SedLine and O3 can be used simultaneously on the same Masimo Root® monitoring hub, making it easier to view and interpret the combined data and thus streamlining our use of the desaturation protocol and workflow during surgery."
Masimo O3 is not currently indicated for somatic use.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Couture EJ, Deschamps A, and Denault AY. Patient management algorithm combining processed electroencephalographic monitoring with cerebral and somatic near-infrared spectroscopy: a case series. Can J Anesth. 2019. https://doi.org/10.1007/s12630-019-01305-y.
2. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
3. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine® and O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine and O3, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Doctella™, a Secure Cloud-based Patient Engagement and Remote Care Automation Platform
Doctella Facilitates Pre- and Post-hospital Care at Home with Customizable, Interactive CarePrograms™ That Utilize Patient-reported and Physiological Data
Irvine, California – February 11, 2019 – Masimo (NASDAQ: MASI) announced today the launch of Doctella™, a home-based patient engagement and remote care automation platform. Doctella provides a complete end-to-end home care solution, allowing clinicians to create and manage treatment plans, patient schedules, and patient data flow using automated, customizable CarePrograms™, home device data aggregation, and a web-based provider dashboard. CarePrograms are delivered to patients' smartphones via an app (available for both iOS® and Android® devices) and dynamically update based on patient input, both self-reported data and physiological data collected by connected monitoring devices.
CarePrograms, configurable via the web-based provider portal, represent a digital, dynamic, and intelligent upgrade to traditional home care plans. Data pushed to patients can include coaching, guidance, and recommendations, such as notifications to remind patients to take medications, connect monitoring devices, or exercise. In turn, the Doctella secure cloud pulls and processes data entered manually by patients – such as patient-reported outcomes and textual responses, with the ability to provide consent through the app – as well as data gathered from connected devices, such as the Masimo MightySat™ Rx fingertip SET® pulse oximeter and the Masimo Rad-97™ rainbow SET™ Pulse CO-Oximeter®, which can act as a Bluetooth®-based hub, capable of pulling in data from a variety of Masimo and third-party devices. Doctella incorporates these data into logic-driven protocols and algorithms that can be customized to meet the needs of clinicians and institutions.
The Doctella provider portal allows clinicians to keep track of various physiological events (such as oxygen desaturations) and behaviors (such as confirming having taken medication as prescribed) for each patient, helping clinicians identify when intervention may be needed and how to prioritize the needs of multiple patients. Through such automation, institutions can more easily deploy home care monitoring at scale while helping clinicians remain abreast of important developments in patient condition. The provider portal can also collect population-level health data to help clinicians gauge the efficacy of various treatment protocols and develop new plans using data-driven decisions and strategy.
Peter Pronovost, MD, PhD, Chief Clinical Transformation Officer at University Hospitals, Ohio, and one of the co-developers of Doctella, said, "As healthcare seeks to improve value, it needs to change its narrative from success being patients healing in the hospital to patients being healthy at home. Doctella helps to accelerate that journey by making sure that patients receive safe, evidence-based care in the healing environment of their own home." Dr. Pronovost recently spoke about the need to change the healthcare narrative at the 2019 World Patient Safety, Science, and Technology Summit.
Patient monitoring at home is an important and increasingly recognized part of providing quality patient care. Home care may be beneficial, for example, for patients with conditions like chronic obstructive pulmonary disease (COPD) and post-surgical patients recuperating at home using prescribed opioids. With proper care pre- and post-surgery at home, patients are expected to do better and help reduce the rate of avoidable hospital readmissions.
Joe Kiani, Founder and CEO of Masimo, said, "Masimo has always sought ways to help caring-clinicians automate and improve the care of their patients. Doctella extends our role into perioperative care outside the hospital and in the home. With customized, automated care plans which funnel patient data to clinicians while helping them prioritize and intervene as needed, Doctella brings clinicians and patients together comprehensively and securely with a complete, end-to-end solution."
Masimo Doctella will be debuted during the HIMSS19 Conference in Orlando, Florida starting February 11, at Masimo’s booth 6259. To schedule a meeting or learn more, please contact [email protected].
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3-5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
4. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Doctella™ and CarePrograms™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Doctella and CarePrograms, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of RRp®, Respiration Rate from the Pleth, on the MightySat™ Rx Spot-Check Fingertip Pulse Oximeter
Masimo MightySat Rx, Now Also Indicated for Use at Home, is the First Fingertip Pulse Oximeter with SET® and the First to Measure Respiration Rate
Irvine, California – January 28, 2019 – Masimo (NASDAQ: MASI) announced today FDA clearance of the measurement of respiration rate from the pleth (RRp®) on the MightySat™ Rx spot-check fingertip pulse oximeter, as well as its indication for use in the home environment. The addition of RRp to MightySat Rx, which also measures functional oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and Pleth Variability Index (PVi®), makes it a more thorough and versatile spot-check solution.
-

Masimo MightySat™ Rx with RRp®
Respiration rate, or the number of breaths taken per minute, typically requires manually counting breaths with a timer and then converting to a rate per minute, or being fitted with chest leads or straps that can be inconvenient. With RRp, respiration rate can conveniently be measured using the same optical sensor that measures SpO2, PR, Pi, and PVi. RRp is provided only when the respiratory movement-induced signal is present in the pulsatile waveform and may not be available during certain conditions, such as very irregular breathing and excessive movement.
With the introduction of RRp to the U.S., Masimo now offers clinicians more ways to accurately measure breathing rate than ever, helping to ensure they have the right tools for each patient scenario. RRp on MightySat Rx offers a convenient, fast, and portable option, adding to the portfolio of respiratory solutions from Masimo that includes continuous, acoustic respiration rate monitoring using RRa® and capnography-based solutions using NomoLine®.
Alongside RRp, PVi, PR, and Pi, MightySat Rx features the same Measure-through Motion and Low Perfusion™ SET® pulse oximetry available in a variety of bedside Masimo and OEM monitors. SET® has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies,1 is estimated to be used on more than 100 million patients a year,2 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.3 The benefits of SET® are maximized by choosing the correct sensor type for each use scenario: adhesive sensors for continuous monitoring, reusable sensors for short-term monitoring, and MightySat Rx fingertip oximeters for spot-check measurements.
MightySat Rx is small and light, weighing less than 100 grams (with batteries), but also durable and long-lasting, with an IP23 ingress rating (protected from water spraying from up to 60 degrees from vertical) and providing approximately 1,800 spot-checks with each set of 2 AAA batteries. Available in both black and white, MightySat offers a high-resolution color display, including display of plethysmographic waveforms, as well as a Bluetooth® wireless interface to the Masimo Professional Health mobile application to track, trend, and communicate measurements.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.6-8 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,2 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.3 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Estimate: Masimo data on file.
3. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview. 4. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
5. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
6. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
7. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
8. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MightySat™ Rx and RRp®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MightySat Rx and RRp, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces U.S. Launch of Iris® Device Management System
Iris Device Management System Facilitates Remote Management of Masimo Point-of-care Devices Throughout the Hospital
Irvine, California – January 22, 2019 – Masimo (NASDAQ: MASI) announced today the U.S. launch of Iris® Device Management System (Iris DMS), an automation and connectivity solution designed to streamline management of Masimo devices used throughout a hospital system.

Masimo Iris®
Device Management System
Iris DMS is designed to address the challenges of maintaining many patient monitors in a complex hospital environment. Iris DMS securely connects over a hospital’s existing network to all connected Masimo devices to provide an easy-to-use dashboard that allows Biomedical Engineers and IT professionals to view detailed diagnostic information about connected Masimo devices at a glance, without the need to physically interact with each device. Iris DMS supports remote software upgrades to ensure all devices stay up to date, easily and efficiently. Patient profiles, policy files, and network settings can be distributed to multiple devices in a single step, simplifying device management. In addition, connected devices routinely "ping" Iris DMS, allowing users to quickly identify any operational issues with any single Masimo device using the intuitive display of near real-time connection status.
Joe Kiani, Founder and CEO of Masimo, said, "More and more clinicians are recognizing the benefits of providing continuous Masimo patient monitoring, not just during surgery or in the ICU, but in all medical-surgical wards, especially for patients on opioids. Masimo patient monitoring devices are more powerful and full-featured than ever, but as a result their software is increasingly complex. Just as with consumer tech devices, medical devices now undergo regular iterative software updates as new innovations and features become available – helping prolong their utility and augmenting their abilities – and as security patches are issued. Iris DMS not only helps Biomedical and IT professionals view detailed diagnostic information about connected Masimo devices at a glance, but helps to simplify the process of maintaining, updating, and standardizing these devices, helping clinicians and hospitals stay focused on providing the best patient care using the most up-to-date technology, not worrying about device maintenance. Iris DMS is another great example of Masimo's pursuit of holistic care solutions that improve patient outcomes and reduce costs across the continuum of care."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3-5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
4. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Iris® Device Management System. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Iris Device Management System, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Select Preliminary Full-Year 2018 Financial Results and 2019 Outlook
Complete fourth quarter and full year 2018 financial results will be announced on Tuesday, February 26, 2019.
- • Preliminary full year 2018 total revenue, including royalty and other revenue, of approximately $855 to $858 million;
- • Preliminary full year 2018 product revenue of approximately $827 to $830 million;
- • Preliminary full year 2018 shipments of noninvasive technology boards and monitors were approximately 232,000;
- • Preliminary full year 2018 GAAP earnings per diluted share and non-GAAP earnings per diluted share are expected to exceed guidance of $3.37 and $2.92 per diluted share, respectively; and
- • Full year 2019 financial guidance includes product revenue, GAAP earnings per diluted share and non-GAAP earnings per diluted share of approximately $910 million, $3.17 and $3.05, respectively.
Irvine, California, January 9, 2019 - Masimo Corporation (NASDAQ: MASI) today announced select preliminary results for the full year December 29, 2018 and provided estimates for its full year 2019 financial guidance.
Preliminary Fourth Quarter 2018 Results:
Masimo expects that its product revenue for the full year 2018 will range from $827 million to $830 million, which reflects growth of 12.0% to 12.4% and constant currency growth of 11.5% to 11.9%. The Company expects its royalty and other revenue for the year will be approximately $28 million. As a result, Masimo now expects its full year 2018 total revenue, including royalty and other revenue, to range from $855 million to $858 million. Additionally, the Company expects that full year 2018 non-GAAP earnings per share will exceed previously issued financial guidance of $2.92, with guidance alone reflecting 27% growth compared to the full year 2017.
The preliminary financial information presented in this press release is based on Masimo’s current expectations and may be adjusted as a result of, among other things, completion of customary annual audit procedures. Management plans to discuss Masimo’s complete fourth quarter and full year 2018 financial results after the market closes on Tuesday, February 26, 2019.
2019 Financial Guidance:
The Company provided the following estimates for its full year 2019 financial guidance:
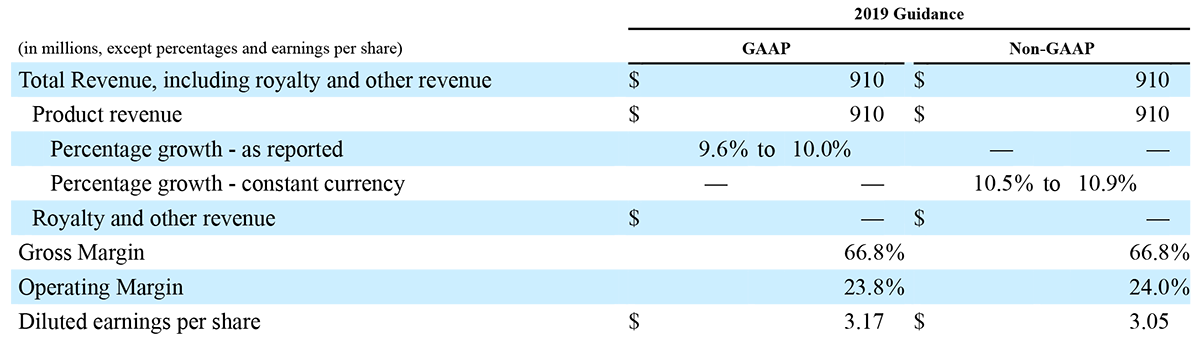
- • Product revenue increasing to $910 million, which reflects reported growth of 9.6% to 10.0% and constant currency growth of 10.5% to 10.9%;
- • GAAP diluted earnings per share increasing to $3.17;
- • Non-GAAP diluted earnings per share increasing to $3.05; and
- • Included in our full year 2019 revenue guidance is approximately $7.0 million of year-over-year currency headwinds.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s ongoing core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an ongoing basis: (i) non-GAAP product revenue growth %, (ii) non-GAAP net income, (iii) non-GAAP diluted earnings per share, (iv) non-GAAP gross profit, (v) non-GAAP operating income and (vi) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP product revenue growth %, non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency adjustments.
Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. These foreign currency revenues, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating our product revenue growth rates on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and the related constant currency adjustments for calculation of our product revenue growth rate will continue to occur in future periods.
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds; most recently in connection with our November 2016 settlement agreement with Koninklijke Philips N.V. We believe that exclusion of these gains and losses is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash.
Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
From time to time, we record tax benefits relating to the derecognition of uncertain tax positions due to the expiration of the statutes of limitations. During the three months ended September 29, 2018, we recorded a significant tax benefit due to the expiration of the applicable statutes of limitations related to certain non-recurring transactions.
We believe that exclusion of the tax benefit resulting from the expiration of certain statutes of limitations related to non-recurring transactions is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that this tax item is unrelated to our core business and non-recurring in nature.
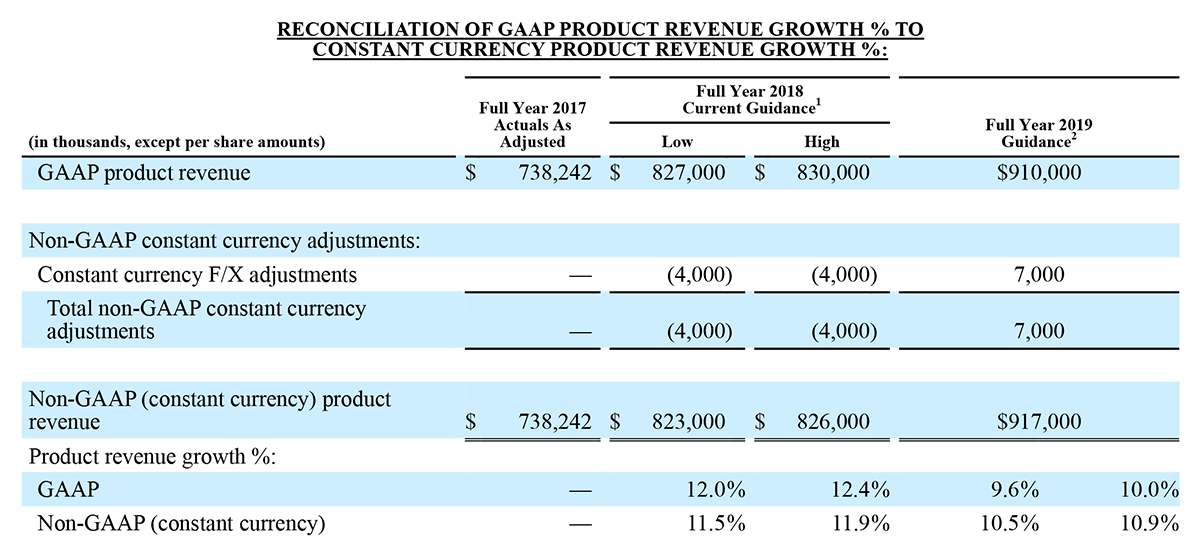

J.P. Morgan Healthcare Conference on Wednesday, January 9, 2019
Masimo will participate in the J.P. Morgan Healthcare Conference on Wednesday, January 9, 2019 at the Westin St. Francis Hotel in San Francisco, California. Micah Young, Executive Vice President and Chief Financial Officer, will represent the Company in a presentation scheduled for 7:30 a.m. Pacific Time.
A live webcast and replay of the Company’s presentation will be available online from the investor relations page of the company’s corporate website at www.masimo.com.
Fourth Quarter and Full Year 2018 Financial Results Conference Call on Tuesday, February 26, 2019
The conference call to review Masimo’s complete financial results for the fourth quarter ended December 29, 2018 will begin at 1:30 p.m. PT (4:30 p.m. ET) on February 26, 2019 and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Micah Young, Executive Vice President and Chief Financial Officer. A live webcast of the conference call will be available online from the investor relations page of the company’s corporate website at www.masimo.com.
The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 6889465. After the live webcast, the call will be available on Masimo’s website through March 26, 2019. In addition, a telephonic replay of the call will be available through March 5, 2019. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 6889465.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2018 and 2019 total, product, royalty and other revenues, earnings per diluted share, operating margin, EBITDA, and estimated tax rate, and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact: Irene Paigah
Phone: (858) 859-7001
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.

Julie A. Shimer Ph.D. Joins Masimo’s Board Of Directors
Irvine, Calif. – January 07, 2019 – Masimo (NASDAQ: MASI) a global medical technology developer and manufacturer of innovative noninvasive patient monitoring technologies, announced today that Julie A. Shimer, Ph.D. has been appointed to Masimo’s Board of Directors.
Dr. Shimer is currently a private investor and has over 30 years of product development experience. Dr. Shimer was President and Chief Executive Officer of Welch Allyn, from March 2007 to April 2012. Prior to Welch Allyn, Dr. Shimer served as President and Chief Executive Officer of Vocera Communications, Inc., from September 2001 through February 2007, also serving on the board of directors. Dr. Shimer also previously held executive positions at 3Com Corporation from January 2000 through August 2001, most recently serving as vice president and general manager of its networking products. Before joining 3Com, she held executive positions at Motorola, Inc., from 1993 through 1999, where she was vice president and general manager for the paging division, and prior to that post, vice president of its semiconductor products section. Dr. Shimer worked for AT&T Bell Laboratories and Bethlehem Steel Company before joining Motorola.
Dr. Shimer is a member of the Board of Directors of Netgear, Inc. (NASDAQ: NTGR), a member of the Board of Directors of Apollo Endosurgery, Inc. (NASDAQ: APEN), a member of the Board of Directors of Windstream Holdings, Corp. (NASDAQ: WIN), and a member of the Board of Directors of Avanos Medical, Inc. (NYSE: AVNS). Dr. Shimer is also a member of the Society of Women Engineers and the Institute of Electrical and Electronics Engineers. Dr. Shimer holds a B.S. degree in Physics from Rensselaer Polytechnic Institute and Master’s and Doctorate degrees in Electrical Engineering from Lehigh University.
“We are delighted to welcome Dr. Shimer to Masimo’s Board of Directors,” said Joe Kiani, Founder, Chairman, and CEO of Masimo. “With her many years of executive leadership and board experience in the medical and communications industries and beyond, as well as her technical expertise and engineering background, she will be an invaluable addition. And I’m particularly excited to have a fellow electrical engineer on our Board.”
“I’m honored to join Masimo’s Board,” said Dr. Shimer. “I’ve long admired Masimo’s commitment to advancing care through innovation and I look forward to contributing to its continued success.”
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3-5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
4. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Iris® Device Management System. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Iris Device Management System, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Investor Contact:
Eli Kammerman
Phone:(949) 297-7077
Email: [email protected]
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
