News & Media-2012
Home / News & Media / 2012
2012
NEWS & MEDIA:
University of North Carolina Hospitals Standardizes to Masimo SET®
Pulse Oximetry for Improved Patient Outcomes
Nationally Recognized Healthcare Organization Installs
Masimo Patient SafetyNet™ for Advanced Patient Monitoring
Chapel Hill, North Carolina & Irvine, California – December 27, 2012 – Masimo (NASDAQ: MASI) today announced that the University of North Carolina Hospitals in Chapel Hill, ranked nationally in three adult and 10 pediatric specialties by U.S. News & World Report (2012-2013), has converted to Masimo SET® pulse oximetry, the standard-of-care at leading hospitals worldwide.
UNC Hospitals joins a growing list of nationally recognized health organizations using Masimo SET®, clinically shown to virtually eliminate false alarms1 and help clinicians detect life-threatening events.2 More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions – helping to substantially contribute to improved patient outcomes.
"We had been using a competitor's pulse oximeter for many years, but I was very familiar with the data demonstrating the superior results of Masimo's technology," said Carolyn Viall Donohue, Associate Chief Nursing Officer and Associate Vice President at University of North Carolina Health Care System. "We were very excited to do a side-by-side comparison with Masimo on patients under anesthesia, and in the ICU and NICU. During the live trial, Masimo performed very well. While Masimo's pulse oximetry is price competitive, this is not just a money issue. Going with Masimo was an improvement in patient care for us."
She recollects an incident in late 2011. Two young boys were admitted to the burn unit with severe inhalation injuries. Their oxygen levels were low, and the staff were unable to maintain pulse oximeter readings on one of the boys. The hospital called a Masimo sales representative and requested a Masimo Radical-7® that the hospital was about to use in a trial of the product. "With the Masimo unit, we got readings on this child when we were not able to get readings from our current pulse oximeter equipment," Viall Donohue said. "The doctors and nurses didn't want to return the (Masimo) pulse oximeter. After this episode the director of respiratory therapy told me, 'I'm a believer in Masimo technology.'"
For 20 straight years, UNC Hospitals – which includes N.C. Memorial Hospital, N.C. Children's Hospital, N.C. Women's Hospital, N.C. Neurosciences Hospital, and N.C. Cancer Hospital – has been included in the U.S. News & World Report Best Hospitals list for multiple specialties. Only 3 percent of hospitals in the United States meet the U.S. News Best Hospitals criteria.
In keeping with its commitment to provide state-of-the-art patient care, UNC Hospitals also is installing Masimo Patient SafetyNet™, which can help ensure patient safety by noninvasively and continuously measuring and tracking a patient's underlying physiological conditions to assist clinicians in detecting changes or abnormalities that signal declining health status. If a patient's condition deteriorates, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response at the patient's bedside. Patient SafetyNet also has been clinically shown to reduce preventable and costly rescue events and transfers to intensive care units. 3
"We are honored to be partners with UNC Hospitals, which has an impressive focus on patient empathy and a well-deserved reputation for providing stellar care," said Masimo founder and CEO Joe Kiani. "By comparing Masimo in side-by-side trials, UNC Hospitals demonstrated its ongoing and data-driven commitment to improving patient safety and care by embracing leading-edge technologies. We share this vision and mission, and look forward to working with UNC Hospitals now and well into the future."
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers". J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study.
3 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
About University of North Carolina Hospitals
UNC Hospitals is an 824-bed public, academic medical center operated by and for the people of North Carolina. The Hospitals' mission is to provide high quality patient care, to educate health care professionals, to advance research and to provide community service. UNC Hospitals includes North Carolina Cancer Hospital, North Carolina Children's Hospital, North Carolina Memorial Hospital, North Carolina Neurosciences Hospital, and North Carolina Women's Hospital. Each year UNC Hospitals cares for patients from all 100 counties in North Carolina and several surrounding states.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: Patient SafetyNet contributes to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Tom Hughes
UNC Health Care System
Phone: (919) 966-6047
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Happy Holidays from Masimo!
Irvine, California — December 21, 2012 — From the launch of iSpO2, our first consumer product, to the release of the breakthrough 2012 Radical-7, this year has been one of great challenges and even greater achievements. As we honor this very special time of the season, it is with gratitude and a heart-felt desire to give something back to a few of the organizations that share our vision for a better world, in your name. Simply respond with an email to [email protected] specifying your charity choice from the list below and we will donate $10 in your  name:
name:
- Amnesty International
- CARE
- Clinton Foundation
- Doctors Without Borders
- Huntington's Disease Society of America
- Make-a-Wish Foundation
- March for Babies
- Masimo Foundation for Ethics, Innovation, and Competition in Healthcare
- Opportunity International
- PATH
- SOS Children's Villages
- Swan Foundation
- Tiyatien Health
- UNICEF
- United Way
- World Vision
As we look to 2013, we are excited to kick off the New Year with the inaugural Patient Safety Science & Technology Summit, Jan. 13-14, featuring former President Bill Clinton as the keynote. We are grateful for the opportunity to have transformed healthcare and we thank each of you for your support in helping us to improve the lives of clinicians and patients we diligently serve. Cheers to a new year full of great possibilities!
Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.®
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
NOTE: Only e-mails sent to [email protected] from official Livewire members will be processed. Please also include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.

Ogden Regional Medical Center Standardizes to Masimo rainbow® Pulse CO-Oximetry™
Leading-Edge Utah Hospital Leverages Advanced Medical Technologies
for Noninvasive, Continuous Patient Monitoring, Including Total Hemoglobin (SpHb®)
Irvine, California – December 19, 2012 – Masimo (NASDAQ: MASI) today announced that Ogden Regional Medical Center (ORMC), named by The Joint Commission as a Top Performer on Key Quality Measures™, has standardized to Masimo rainbow® Pulse CO-Oximetry™ and sensor technologies for advanced patient monitoring. This noninvasive technology helps save lives by making it safer, faster, and easier than ever for healthcare professionals to measure and monitor patient conditions – on-the-spot, in just seconds.
The conversion equips Ogden Regional Medical Center with breakthrough rainbow® technology. Among its multiple capabilities, this innovation enables needleless and pain-free blood testing of total hemoglobin (SpHb®) – and delivers immediate results for hospital patients.
"I like the fact that you can pinpoint the total hemoglobin in real time, and quickly identify a patient's status on a waveform display; both of these features make my job more manageable," said Travis Slade, MD, an anesthesiologist at ORMC. "I've also been very impressed with the device's sensitivity. It consistently displays results that are nearly identical to (hemoglobin) lab tests we've run. It also works when other technologies don't. There have been cases when we used a conventional pulse ox that didn't produce a SpO2 reading. We brought in the Masimo, and got the results we needed."
Other Masimo rainbow® measurements in use at ORMC include SpCO®, which helps clinicians to more quickly detect elevated levels of carbon monoxide in the blood – which can facilitate earlier diagnosis and treatment for patients poisoned by carbon monoxide; and Pleth Variability Index (PVI®), which can continuously assess and allow clinicians to better manage fluids as they are administered to patients – in a noninvasive and cost-effective manner.
As part of its system-wide conversion, ORMC will also leverage Masimo SET® pulse oximetry in every patient care setting. A standard-of-care at leading hospitals worldwide, Masimo SET® has been clinically shown to virtually eliminate false alarms1 and increase a clinician's ability to detect life-threatening events.2
"We are thrilled that ORMC, with its nationally recognized reputation for quality of care, has selected Masimo SET® and Masimo rainbow® technologies as key parts of its ongoing commitment to patient safety," said Masimo CEO and founder Joe Kiani. "It is an honor to be able to partner with such an esteemed healthcare organization, and we look forward to working with ORMC for years to come."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
About Ogden Regional Medical Center
This MountainStar Healthcare hospital utilizes advanced technologies while delivering nationally recognized care to patients in northern Utah and surrounding communities. It was recently honored with the 2012 100 Top Hospitals® Award in the category of medium-sized community hospitals and recognized as a 2011 Top Performer on Key Quality Measures™ for heart attack care, pneumonia and surgical care by The Joint Commission. It is one of only three in Utah to achieve the 2012 and 2011 Maternity Care Excellence Award from HealthGrades, a national healthcare rating organization, which also recognized the hospital among the top fifteen percent in the country for treating COPD and sepsis. As a Level II Trauma Center and a Certified Stroke Center, ORMC was ranked among the top 15 percent in the nation for stroke treatment in 2011 by HealthGrades.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb), SpOC, SpCO, SpMet, and PVI contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at 31st Annual J.P. Morgan Healthcare Conference
IRVINE, Calif., December 18, 2012 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 31st Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco on Tuesday, January 8, 2013, at 1:30 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Launches iSpO2™ – Commercially Available Pulse Oximeter for iPhone, iPad & iPod touch
Irvine, California – December 13, 2012 – In keeping with its commitment to excellence and innovation, Masimo (NASDAQ: MASI) today announced the debut of the iSpO2™ pulse oximeter cable and sensor with Measure-Through Motion and Low Perfusion Masimo SET® technology for use with iPhone, iPad or iPod touch with 30-pin connector.
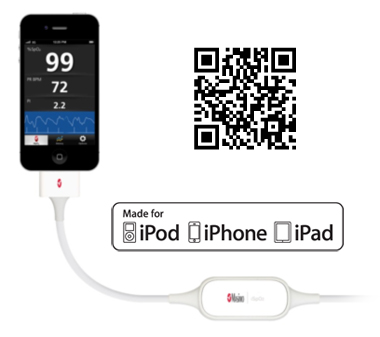
- CAPTION: Masimo iSpO2™ pulse oximetry cable and sensor (available at iSpO2.com) is compatible with the iPhone, iPad, or iPod touch
iSpO2™ uses the same technology found in Masimo's breakthrough line of pulse oximeters and Pulse CO-Oximeters™ – the standard-of-care pulse oximetry technology at work in leading hospitals around the world – providing accurate measurements, even during the challenging conditions of motion and low perfusion.
Customers can purchase the iSpO2™ Signal Extraction Pulse Oximeter and use their iPhone, iPad or iPod touch to check their own blood oxygenation (SpO2), pulse rate, and perfusion index measurements for sports and aviation use and is not intended for medical use.
After purchasing the iSpO2™ (available at iSpO2.com and Amazon) and connecting it to an iPhone, iPad or iPod touch, the iSpO2™ application will automatically download. Thereafter, using the iSpO2™ is easy as 1-2-3:
- Connect the iSpO2™ cable to your iPhone, iPad or iPod touch
- Slip the iSpO2™ sensor on your ring finger
- Your results appear on screen
The iSpO2™ Medical, the professional version for medical use, is pending CE Mark and U.S. FDA 510(k) clearance. This product will be made available through Masimo's existing distribution channels.
"Masimo was founded on a mission to take noninvasive monitoring to new sites and applications," said Masimo founder and CEO Joe Kiani. "The iSpO2™ for iPhone, iPad or iPod touch with 30-pin connector represents our first consumer product and should create myriad new possibilities with the goal of further empowering people."
Please visit iSpO2.com for purchasing information.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the new iSpO2, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium

VOTE EARLY – VOTE OFTEN Voting for the first REAL Awards is now open!
In September, we were proud to announce our involvement in the first ever REAL Awards – an awards series dedicated to the inspiring, and often life-saving, work of health workers around the world. Since the launch of this initiative, hundreds of nominations were submitted on behalf of caring and dedicated health workers right here in the U.S.
And now is the best part – reading the stories! By visiting www.theREALawards.com, you can read the heartfelt stories about health workers nominated in eight different categories:
- Newborn and Mother care
- Pediatric care
- Chronic Disease care
- Hospice care
- Emergency care
- Veteran care
- At-home care
- General Health Worker
You can vote once a day, every day, until the voting period ends on January 7.
Please visit www.theREALawards.com today and cast your vote for a truly deserving health worker – and don't forget to encourage your friends and family to take part in this important and uplifting initiative by sharing your votes on twitter and Facebook!

Saint Mary's Healthcare Standardizes on Industry-Leading Masimo SET® Pulse Oximetry
Nationally Recognized Hospital Also Adds Masimo rainbow® Pulse CO-Oximetry for Noninvasive Patient Monitoring of Total Hemoglobin (SpHb®) and Carboxyhemoglobin (SpCO®)
Amsterdam, New York & Irvine, California – November 29, 2012 –Saint Mary's Healthcare, the first hospital in New York State to achieve the "Pathway to Excellence" Designation from the American Nurses Credentialing Center, andMasimo (NASDAQ: MASI) today jointly announce that Saint Mary's has standardized to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, the standard-of-care at leading hospitals worldwide.
Standardizing to Masimo SET® allows clinicians to accurately monitor blood oxygen saturation in challenging conditions – establishing the technology as an industry-leading pulse oximetry that substantially contributes to improved patient outcomes.
"The one thing I really appreciate about Masimo's technology is that we've been able to get dependable, reliable readings on neonates," said Rob Noel, Senior Respiratory Therapist at Saint Mary's Healthcare. "We're getting more readings than we were before, especially when movement is involved. When we put this technology on newborns, we know we're getting the right information."
In addition to Masimo SET®, which virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events,2 Saint Mary's will benefit from breakthrough rainbow® technology that allows clinicians to measure multiple blood constituents, fluid responsiveness, respiration rate, and other physiological parameters noninvasively and continuously. Parameters at Saint Mary's include total hemoglobin (SpHb®), and carboxyhemoglobin (SpCO®), as well as the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
Saint Mary's will deploy SpHb in its emergency department. Masimo SpHb enables clinicians to measure hemoglobin and trending noninvasively and continuously.3 A recent study also noted that, "Because SpHb measurement is noninvasive, it has the potential to confer the additional benefits of patient comfort, decreased complexity, and increased safety for health care providers who are not exposed to risks of needle-stick injury and blood-spill contaminations."4 Likewise, SpCO allows clinicians to noninvasively and quickly detect elevated levels of carbon monoxide in the blood – facilitating earlier diagnosis and treatment.5
According to Peter Holtermann, Director of Medical Imaging at Saint Mary's, "The true benefit of the standardized equipment is to the patient. The accuracy of the testing and its less invasive nature enable patients to receive care that is less painful and more timely."
"We are happy to have this partnership with Saint Mary's, which like other great healthcare organizations is demonstrating its commitment to patient safety and care by taking advantage of our breakthrough patient monitoring technologies such as SpHb – a continuous noninvasive way to measure total hemoglobin, and our measure-through motion and low perfusion pulse oximetry technology, which has been proven to improve care and reduce costs," said Masimo founder and CEO Joe Kiani. "Together, we look forward to helping Saint Mary's meet and exceed its patient-care needs."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
3 Frasca D., Dahyot-Fizelier C., Catherine K., Levrat Q., Debaene B., Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit Patients." Crit Care Med. 2011 Oct;39(10):2277-82. http://www.masimo.com/pdf/clinical/hemoglobin/frasca-accuracy-of-a-continuous-noninvasive-hemoglobin-monitor-Crit-Care-Med-2011.pdf.
4 Raikhel M. "Accuracy of Noninvasive and Invasive Point-of-Care Total Blood Hemoglobin Measurement in an Outpatient Setting" Postgraduate Medicine, Volume 124, Issue 4, July 2012, ISSN – 0032-5481, e-ISSN – 1941-9260.
5 Hampson N. "Noninvasive pulse CO-oximetry expedites evaluation and management of patients with carbon monoxide poisoning." The American Journal of Emergency Medicine 2012 (10.1016/j.ajem.2012.03.026) http://www.sciencedirect.com/science/article/pii/S0735675712001301
About Saint Mary's Healthcare
St. Mary's Healthcare is a continuum of health care services offering a comprehensive network of high quality, local, compassionate care. Services include but are not limited to 120 acute care beds, 10 primary and specialty care centers in Fulton and Montgomery counties, more than 30 behavioral health services in Fulton, Montgomery and Hamilton counties, a 10 bed Acute Inpatient Rehabilitation Unit, a 10 bed Transitional Care Unit, and a 160-bed nursing home. St. Mary's received 5-star ratings from HealthGrades for four consecutive years and in 2011 became the first hospital in New York State to achieve the "Pathway to Excellence" Designation. Our 1600+ associates embrace the mission of providing every individual with an exceptional patient experience. Founded by the Sisters of St. Joseph of Carondelet in 1903, and as a member of Ascension Health since 2002, St. Mary's is dedicated to improving the health of the community with special attention to the poor and underserved. Please visit www.smha.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb) and SpCO contribute to positive clinical outcomes and patient safety under all circumstances; risks related to our belief that Masimo noninvasive medical breakthroughs provide solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jerri Cortese
Saint Mary's Healthcare
Phone: (518) 841-7162
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

IWK Health Centre Standardizes to Masimo SET® Pulse Oximetry
Leading-Edge Canadian Hospital Deploys Breakthrough Measure-Through Motion and Low Perfusion Pulse Oximetry Technology
Irvine, California – November 27, 2012 – Masimo (NASDAQ: MASI) today announced that the IWK Heath Centre, Atlantic Canada's leading health centre providing specialized, quality care to women, children, youth and families, has converted to Masimo SET® pulse oximetry and sensor technologies, the standard-of-care at leading health organizations worldwide.
Masimo SET® pulse oximetry virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events2 – helping to substantially contribute to improved patient outcomes and patient safety. Masimo SET® pulse oximetry also has been shown to help clinicians reduce retinopathy of prematurity3, screen for critical congenital heart disease in newborns4, and save lives in post-surgical floors, recovery2, labor and delivery rooms5, and ICUs6.
IWK's conversion to Masimo SET® pulse oximetry is in keeping with the IWK's dedication to excellence in care and delivering the best possible results for its patients. More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions, including patient movement and low peripheral perfusion.
"Specializing in Children's and Women's Health, it is vital that we use the technology that provides our patients and staff with a consistent and accurate measure of blood oxygen saturation levels," said David Hancock, Technology Assessment Officer, IWK Health Centre. "We have standardized on Masimo technology in our ICUs, ORs and inpatient care areas for all patient monitoring, which will lead to improved patient outcomes."
The Health Centre also has the option to upgrade at any time to Masimo rainbow® Pulse CO-Oximetry™, which allows clinicians to noninvasively monitor multiple blood constituents that previously required invasive or complicated procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™).
"We are happy to have forged this great partnership with the IWK, which like other leading healthcare organizations is demonstrating its commitment to patient safety and care by taking advantage of our breakthrough measure-through motion and low perfusion pulse oximetry technology," said Masimo founder and CEO Joe Kiani. "We value this relationship with IWK, and together look forward to helping their skilled and dedicated staff meet and exceed their patient-care needs."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
3 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4 de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
5 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518.http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2010.02097.x/abstract
6 Goldstein MR, Martin GI, Sindel BD, Furman GI, Ochikubo C, Yand L. SatSeconds Alarm Management Misses Short Desaturations Common to Periodic Breathing and Infantile Apnea. Pediatric Research 2001;49(4):400A/2296.
About the IWK Health Centre
The IWK Health Centre is Atlantic Canada's leading health centre providing specialized, quality care to women, children, youth and families in the Maritimes and beyond. In addition to providing highly specialized (tertiary) care, the IWK also provides primary care services. The IWK is also engaged in leading-edge research; works to promote healthy lifestyles for families; and as an academic health centre provides education opportunities for health professionals and other learners. Visit www.iwk.nshealth.ca for more information.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb), SpCO, SpMet, PVI, and RRa contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Nick Cox
IWK Health Centre
Phone: (902) 470-6740
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Corporation Founder and CEO Joe Kiani Named Ernst & Young National Entrepreneur Of The Year – 2012 Life Sciences Award Winner Joe Kiani honored for revolutionizing today's health care technologies with the invention and commercialization of noninvasive patient monitoring devices
 IRVINE, California, November 20, 2012 – Joe Kiani, founder, CEO and Chairman of the Board of Masimo Corporation, has been named the Ernst & Young National Entrepreneur Of The Year – 2012 Life Sciences Award Winner. The Ernst & Young Entrepreneur Of The Year Award is the country's most prestigious business award for entrepreneurs. The award encourages entrepreneurial activity and recognizes leaders and visionaries who demonstrate innovation, financial success and personal commitment as they create and build world-class businesses.
IRVINE, California, November 20, 2012 – Joe Kiani, founder, CEO and Chairman of the Board of Masimo Corporation, has been named the Ernst & Young National Entrepreneur Of The Year – 2012 Life Sciences Award Winner. The Ernst & Young Entrepreneur Of The Year Award is the country's most prestigious business award for entrepreneurs. The award encourages entrepreneurial activity and recognizes leaders and visionaries who demonstrate innovation, financial success and personal commitment as they create and build world-class businesses.
Kiani was recognized for revolutionizing the health care industry by taking risks to create and commercialize noninvasive patient monitoring devices, which include a wide array of sensors that lead to improved accuracy, a reduction in the overall number of false readings, and ultimately, reduced cost of care. Kiani was honored at the Entrepreneur Of The Year Awards gala, the culminating event of the Ernst & Young Strategic Growth Forum in Palm Springs, Calif. The Forum is the nation's premier gathering of high-growth, market-leading companies. Awards were given in nine additional categories. The Ernst & Young Entrepreneur Of The Year Award winners were selected by an independent panel of judges from 244 regional award recipients.
"We are pleased to honor Joe Kiani with this esteemed award and recognize him for his perseverance, passion, and innovative mind – all of which continue to make a positive impact on health care and patient monitoring technologies," said Bryan Pearce, Americas Director, Entrepreneur Of The Year, Ernst & Young LLP. "Joe's innovative approach, discipline and triumph over the years truly demonstrate the meaning of the entrepreneurial spirit we've come to celebrate over the last 26 years."
The early rise and collapse
By training, Kiani is an electrical engineer, graduating from high school at the mere age of 15. Kiani's entrepreneurial spirit sprouted early on at his first job, a smaller company with pulse oximeter technology, where he worked in exchange for stock – not cash. Kiani's aptitude and contributions led to his swift rise to the top as president of the company, after its upcoming merger, at an early age. But as hasty as the rise, Kiani quickly learned an important aspect of ethics and integrity. As a result of his efforts in honesty, Kiani was blamed for the collapse of the company's pending merger. This decision and outcome ultimately led to Masimo Corporation.
Passion & discipline: never settle for less
With Kiani's developed passion for adaptive signal processing for noninvasive monitoring, he took the money he had saved and a $40,000 bank loan and began to develop noninvasive patient monitoring technology from his garage. Within three short years of maintaining a strong financial discipline, Kiani had raised substantial capital and was well on his way to creating medical technology that has transformed the health care industry today – technology that would not exist were it not for Masimo Corporation.
Despite hospital administrators and clinicians embracing Masimo's superior technology, Kiani still faced barriers. Larger, more established companies and group purchasing organizations (GPOs) were reluctant to let Masimo Corporation succeed in the market. Kiani stuck by his belief of always doing what is right for patient care and emerged victorious after Senate investigations into anticompetitive practices of GPOs and established companies, including his main rival, where Kiani testified twice in front of the U.S. Senate Judiciary Committee, and a seven-year patent infringement legal battle – a battle that threatened to bankrupt Masimo.
Giving back and thriving
As Masimo emerged as a success story in the medical industry, many advised Kiani to buy out his existing shareholders. But instead, he distributed much of his profit in a special dividend and offered a substantial appreciation bonus to employees. Soon after, Kiani knew Masimo could achieve bigger and better things, and in order to do so, he took the company public. In its first five years as a public company, Masimo Corporation product revenues increased by more than 100 percent, and net income increased by more than 175 percent. Throughout it all, Kiani never stopped taking risks to grow what is now a multi-million dollar public company with more than 2,700 employees.
About Ernst & Young's Entrepreneur Of The Year Ernst & Young's Entrepreneur Of The Year is the world's most prestigious business award for entrepreneurs. The unique award makes a difference through the way it encourages entrepreneurial activity among those with potential and recognizes the contribution of people who inspire others with their vision, leadership and achievement. As the first and only truly global award of its kind, Entrepreneur Of The Year celebrates those who are building and leading successful, growing and dynamic businesses, recognizing them through regional, national and global awards programs in more than 140 cities in 50 countries.
About Ernst & Young
Ernst & Young is a global leader in assurance, tax, transaction and advisory services. Worldwide, our 167,000 people are united by our shared values and an unwavering commitment to quality. We make a difference by helping our people, our clients and our wider communities achieve their potential.
For more information, please visit ey.com.
SOURCE Ernst & Young

Maricopa Integrated Health System Installs Masimo Patient SafetyNet™ System for
Improved Oversight of General Floor
Installation Is Part of an Organization-Wide Conversion to Masimo SET® Pulse Oximetry and
rainbow® Pulse CO-Oximetry for Advanced Patient Monitoring
Livewire (Internal Only) Phoenix, Arizona & Irvine, California – November 15, 2012 – Maricopa Integrated Health System, among the most trafficked hospital systems in Arizona with more than 500 licensed beds, andMasimo (NASDAQ: MASI) today announced that Maricopa has deployed Masimo Patient SafetyNet™, clinically shown to help improve patient outcomes and save money.1
The installation is part of the nationally recognized healthcare organization's standardization to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry and rainbow® SET® Pulse CO-Oximetry.
Maricopa joins a growing list of leading health systems using Patient SafetyNet, which can help ensure patient safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and detect changes or abnormalities that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
Maricopa patients also will benefit from breakthrough rainbow® technology that allows clinicians to measure multiple blood constituents, respiration rate, and other physiological parameters without invasive blood draws and time-consuming laboratory analysis. Parameters in use include total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The pulse oximetry standard-of-care at leading hospitals worldwide, Masimo SET® virtually eliminates false alarms2 and increases a clinician's ability to detect life-threatening events.3
Blood transfusions carry risks, including a significant link to mortality, infection, and adult respiratory distress syndrome.4 Masimo's SpHb has been clinically shown to help anesthesiologists reduce the frequency of blood transfusions.5
"I've found total hemoglobin (SpHb) to be particularly useful when I'm in the OR because it offers a continuous measurement," said William Johnson, MD, Chairman of the Department of Anesthesiology at Maricopa Medical Center. "In cases where there's insidious blood loss during some of the longer surgeries, you can keep track of the trend. And when there's relatively rapid loss, the Masimo monitor produces a graph to show hemoglobin falling. In either of those extremes, the ability to track blood loss may make you more inclined not to give a unit of blood when you can predict hemodynamics. When used properly, SpHb can reduce the urge to give blood when it's not absolutely necessary, which can improve patient safety and outcomes."
Masimo founder and CEO Joe Kiani stated: "As studies have shown, SpHb continuously monitors patient hemoglobin levels to help clinicians better govern when – and more importantly, when not – to transfuse. Because blood represents a major cost for hospitals and unnecessary transfusions can be bad for patients, SpHb can offer better patient care at a lower price. Likewise, the interoperability of our physiological parameters with Patient SafetyNet in the general ward also can help contain costs through early detection of adverse events, which can reduce preventable and costly transfers to intensive care units. We congratulate Maricopa Integrated Health System for its dedication to cost-effective patient safety."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
2 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
4 Marik, P. E. and H. L. Corwin (2008). "Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature." Crit Care Med 36(9): 2667-74.
5 Ehrenfeld JM, Henneman JP, Sandberg WS. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." American Society Anesthesiologists. 2010;LB05
About Maricopa Integrated Health System
Maricopa Integrated Health System (MIHS) is the public health care system for the State of Arizona. MIHS includes Maricopa Medical Center, the Arizona Burn Center, the Arizona Children's Center, the Arizona Cancer Center, and eleven Family Health Centers located throughout Maricopa County. MIHS also includes two behavioral health centers and an attendant care program. For more information, please visit www.mihs.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Maricopa Integrated Health System patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Trinity Medical Center Adds Masimo Patient SafetyNet™ as a High
Vigilance Safeguard for Post-Surgical Patients
Leading Alabama Hospital Leverages Masimo SET® Pulse Oximetry for Oxygenation Monitoring
and Masimo rainbow® Acoustic Monitoring for Ventilation Monitoring
Birmingham, Alabama & Irvine, California – November 12, 2012 – Masimo (NASDAQ: MASI) and Trinity Medical Center announced today the successful installation of Masimo Patient SafetyNet™, clinically shown to reduce rapid response activations and intensive care unit (ICU) transfers, while offering significant potential cost savings.1
Trinity joins a growing list of outstanding hospitals using Patient SafetyNet to track the underlying physiological conditions of post-surgical patients and detect changes or abnormalities that signal declining health status in real-time. When a patient's condition deteriorates, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
Trinity also is using rainbow® Acoustic Monitoring, which noninvasively and continuously measures a patient's respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck. Acoustic Respiration Rate (RRa™) is a breakthrough measurement that facilitates earlier detection of respiratory compromise and patient distress – especially important for post-surgical patients receiving patient-controlled analgesia for pain management, as the sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.2-4
"Incorporating new technologies plays a key role in nurturing our culture of patient safety," said Keith Granger, President and CEO of Trinity Medical Center. "Our clinicians are finding this equipment to be a valuable tool as we work to continue to improve patient care."
Patient SafetyNet can help hospitals comply with The Joint Commission's Sentinel Event Alert released earlier this year that focused on the recommendation to continuously monitor post-surgical patients for possible adverse opioid effects.5 Fortunately, improved care does not need to come at a higher cost. Clinicians at Dartmouth-Hitchcock Medical Center in New Hampshire reported that using the Masimo Patient SafetyNet system on just one floor reduced ICU transfers and created an annual opportunity-cost savings of $1.48 million.1
Joe Kiani, Chairman and CEO of Masimo, stated: "We are happy that our breakthrough technologies help Trinity Medical Center's dedicated staff provide their patients with the best care possible. Each day we work to improve patient safety and reduce healthcare costs. With Masimo technologies on the general floor, clinicians can be confident their patients are being safely monitored even when they aren't at the bedside."
Trinity Medical Center has been named one of the nation's Top Performers on Key Quality Measures by The Joint Commission, the leading accreditor of health care organizations in the United States. The hospital was recognized by The Joint Commission for exemplary performance in using evidence-based clinical processes that are shown to improve care for certain conditions, including heart attack, heart failure, pneumonia, surgical care, children's asthma, stroke and venous thrombo embolism.
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
2 Joint Commission on Accreditation of Healthcare Organizations. Sentinel event alert: patient controlled analgesia by proxy; JCAHO. 2004.
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part I – How errors occur; ISMP. 2003.
4 Bird M. Acute pain management: a new area of liability for anesthesiologists; ASA Newsletter. 2007; 71:8.
5 The Joint Commission. Sentinel event alert: Safe use of opioids in hospitals; 2012
About Trinity Medical Center
Trinity Medical Center is a tertiary care hospital serving residents of Birmingham, Alabama, and surrounding communities. The hospital offers programs and services in all medical and surgical areas. This includes oncology, cardiology, robotic surgery, orthopedics, neurology, women's health, mental health, digestive diseases, geriatrics, physical medicine and rehab, sports medicine, cardiac and pulmonary rehab, and emergency care. Trinity Medical Center has been named one of the nation's Top Performers on Key Quality Measures by The Joint Commission, the leading accreditor of health care organizations in the United States. The hospital was recognized by The Joint Commission for exemplary performance in using evidence-based clinical processes that are shown to improve care for certain conditions, including heart attack, heart failure, pneumonia and surgical care. For more information about Trinity Medical Center visit www.trinitymedicalonline.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: acoustic respiration rate (RRa™) and Patient SafetyNet contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Leisha Harris
Trinity Medical Center
(205) 599-4926
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Skagit Valley Hospital Standardizes on Masimo SET®
Measure-Through Motion and Low Perfusion Pulse Oximetry
Dedicated to Patient Safety, Hospital Also Will Deploy Masimo's Breakthrough Noninvasive rainbow®Acoustic Monitoring Technology for Advanced Respiration Rate Monitoring
Mount Vernon, Washington and Irvine, California – November 6, 2012 – Skagit Valley Hospital, among the Top 100 in 2012 for joint replacement, orthopedics and prostatectomy, according to health care ratings firm HealthGrades, and Masimo (NASDAQ: MASI) today announced the hospital's system-wide standardization to Masimo SET®Pulse Oximetry.
Skagit Valley Hospital will standardize on SET pulse oximetry technology at every patient bed and in every care area(monitors and sensors), creating an environment that provides Skagit Valley Hospital clinicians with accurate patient monitoring capabilities during challenging conditions, including patient movement and low perfusion.
"Skagit Valley Hospital is dedicated to providing safe, quality care to our patients. We are pleased to continue to bring in the latest in technology to benefit the patients we serve," said Getty Phippen, Skagit Valley Hospital Director of Pulmonary and Sleep Services. "Masimo's technology is excellent and the monitors are precise, picking up signals even when patients' hands are cold."
Skagit Valley Hospital will also deploy rainbow®Acoustic Monitoring for acoustic respiration rate (RRa™), which noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck. RRa is a breakthrough measurement that facilitates earlier detection of respiratory compromise and patient distress – especially important for post-surgical patients receiving patient-controlled analgesia for pain management, as the sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.1-3
"We compared Masimo to others and found Masimo's technology works better, especially when patients moved around a lot," said Bill Thomas, Materials Management Director at Skagit Valley Hospital. "We're happy with the product and it cost less. So, we're saving money and getting a better outcome for our patients. It was a win-win."
"This partnership with Skagit Valley Hospital is another example of a leading healthcare organization improving patient outcomes with Masimo SET®technology," said Joe Kiani, chairman and CEO of Masimo. "Patient care and safety remain our top priority, and we're happy that our technology will help Skagit Valley Hospital keep continuous and noninvasive watch over their patients, whether in the OR or on the general floor."
Masimo SET®virtually eliminates false alarms4 and helps clinicians detect life-threatening events.5 More than 100 independent clinical studies have confirmed that Masimo SET®technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions – helping to substantially contribute to improved patient outcomes. The option to upgrade to Masimo rainbow®SET®Pulse CO-Oximetry allows clinicians to noninvasively monitor multiple blood constituents that previously required invasive or complicated procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa).
1 The Joint Commission. Sentinel event alert: Safe use of opioids in hospitals; 2012.
2 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part I – How errors occur; ISMP. 2003.
3 Bird M. Acute pain management: a new area of liability for anesthesiologists; ASA Newsletter. 2007; 71:8.
4 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
5 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
About Skagit Valley Hospital
Licensed for 137 beds, Skagit Valley Hospital is a regional, public facility serving residents of Skagit, Island, San Juan and north Snohomish counties since 1958. The Mount Vernon hospital and Skagit Regional Clinics, with eight locations, are part of the integrated system known as Skagit Regional Health. The hospital's comprehensive services include the Skagit Regional Heart & Vascular Institute, a Level III Trauma Center, a comprehensive Regional Cancer Care Center, the well-appointed Family Birth Center and surgical services with a focus on orthopedics and minimally invasive procedures. For more information, visit http://www.skagitvalleyhospital.org/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb), SpOC, SpCO, SpMet, PVI, and RRa, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Kari Ranten
Skagit Regional Health
Phone: (360) 814-2370
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Reports Third Quarter 2012 Financial Results; Board Declares Special Cash Dividend of $1.00 per Share
Q3 2012 Highlights (compared to Q3 2011):
- Product revenue rose 15% to $112.1 million
- Masimo rainbow® revenue rose 41% to $11.0 million, including 92% increase in SpHb® revenue
- Shipped 33,100 Masimo SET® and Masimo rainbow® SET units
- Net income was $13.8 million, with EPS of $0.24
Irvine, California, November 1, 2012 – Masimo (NASDAQ: MASI) today announced its financial results for the third quarter ended September 29, 2012, and that its Board of Directors has declared a special cash dividend of $1.00 per share, payable on December 11, 2012 to stockholders of record as of the close of business on November 27, 2012.
Masimo's total third quarter revenue, including royalties, rose 14% to $119.1 million, compared to $104.0 million for the third quarter of 2011. Third quarter 2012 product revenue rose 15% to $112.1 million, compared to $97.6 million for the third quarter of 2011. The company's worldwide end-user revenue grew 15% in the third quarter of 2012 and represented 85% of product revenue. OEM sales, which accounted for 15% of product revenue, rose 13% compared to the same period in 2011. Excluding the impact of Masimo's 2012 acquisitions of PHASEIN AB and Spire Semiconductor, third quarter 2012 product revenue rose 12%. Revenue from sales of Masimo rainbow products rose 41% to $11.0 million in the third quarter, compared to $7.8 million for the third quarter of 2011. Third quarter 2012 rainbow revenue included a 92% increase in total hemoglobin (SpHb) sales compared to the third quarter of 2011.
Net income for the third quarter of 2012 was $13.8 million, or $0.24 per diluted share, compared to net income of $14.8 million, or $0.24 per diluted share, in the third quarter of 2011. The third quarter 2012 operating results included a $0.02 per share loss attributed to Masimo's 2012 acquisitions of PHASEIN AB and Spire Semiconductor.
During the third quarter of 2012, the company shipped approximately 33,100 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, essentially unchanged from the same prior-year period. Masimo estimates its worldwide installed base as of September 29, 2012 to be 1,056,000 units, up 11% from 950,000 units as of October 1, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "During the third quarter, we continued to leverage Masimo's innovation leadership and recurring-revenue business model to seize multiple growth opportunities across a range of patient care settings. Our third quarter product revenue and global installed base growth continued to outpace the industry, and third quarter rainbow product sales were among the highest yet."
Mr. Kiani continued, "We are happy to announce the Board's decision to declare a special cash dividend, which reflects not only the strength of our financial position and confidence in our long-term outlook, but also demonstrates our commitment to enhance stockholder value. In addition, by declaring the dividend now, Masimo stockholders can take advantage of current dividend tax rates, which may increase in 2013. Today's action marks the fourth instance of a special dividend paid by Masimo in the past six years, and the third since we became a public company in 2007. In fact, including the dividend announced today, since becoming a public company, we have returned approximately $220 million to stockholders in the form of special cash dividends and another $62.5 million in the form of repurchases of our common stock. This total of $282.5 million represents a return of approximately 88% of the cash generated from operations between 2008 and September 2012."
As of September 29, 2012, Masimo's cash and cash equivalents were $113.0 million, compared to $129.9 million as of December 31, 2011. The change reflects primarily net cash generated from operations, offset by $37.4 million in cash used to acquire PHASEIN AB and Spire Semiconductor, and $26.3 million in cash used to repurchase shares of Masimo common stock in the first half of 2012. The payout for the special $1.00 per share cash dividend announced today is expected to be about $57.2 million, based on the current shares outstanding, and will be reflected in the company's fourth quarter and full year 2012 financial statements. The special dividend payout represents only a portion of the company's cash reserves, which the Board believes is sufficient to cover operational needs, and fund continued research and development investments and strategic initiatives.
While dividends are not routine for the company, Masimo has previously paid special dividends to shareholders totaling $4.09 per share related to the 2006 intellectual property patent suit settlement agreement with a competitor, $2.00 per share in March 2010 and $0.75 per share in December 2010. Although there can be no guarantees of future dividends, the Board remains committed to enhancing stockholder value based on its consideration of various factors, including the company's operating results, financial condition and anticipated capital requirements.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 38439591. After the live webcast, the call will be available on Masimo's website through December 1, 2012. In addition, a telephonic replay of the call will be available through November 15, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 38439591.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Media Contact:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

Premier Signs SedLine® Supplier Agreement with Masimo Contract Offers Member Hospitals Direct Access to SedLine Brain Monitoring System to Help Enhance Anesthetic Control and Better Manage All Phases of Anesthesia
Irvine, California – November 1, 2012 – Masimo (NASDAQ: MASI) announced today it has entered an agreement with Premier, a leading national healthcare group purchasing organization, for Masimo's SedLine® brain function monitoring system. Masimo was awarded the agreement through a competitive bid, marking the first time Premier will offer SedLine to its members.
SedLine technology advances neuromonitoring capabilities to help improve the care of patients under anesthesia or sedation. By continuously measuring brain activity on both sides of the brain simultaneously and providing real-time information about a patient's response to anesthesia, SedLine helps the anesthesia provider deliver the desired level of targeted sedation throughout all phases of anesthesia – in the OR and ICU. In addition to offering dependable monitoring during challenging conditions such as electrocautery, SedLine's unique Density Spectral Array enables immediate detection of asymmetrical activity. Use of SedLine and its Patient State Index™ (PSI) has been shown to help clinicians manage patients to significantly faster emergence from anesthesia and faster recovery.1
Masimo President of Worldwide Sales, Marketing and Clinical Research, Jon Coleman, stated, "We are happy that Premier recognizes the utility of our high fidelity brain function monitor to measure the varying stages of consciousness for sedated patients. Masimo's SedLine brain function monitoring system offers the ability to continuously monitor a patient's level of anesthesia and sedation in the OR and ICU settings, helping clinicians titrate anesthesia properly."
Effective through July 31, 2015, Premier members may purchase Masimo SedLine brain function monitoring through Masimo's direct sales and clinical education team. For more information on Masimo SedLine, contact Jim Beyer, Vice President of Corporate Sales, at [email protected].
1 Drover DR, Lemmens HJ, Pierce ET, et al. Patient State Index: titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia.Anesthesiology. 2002;97:82-89.
About the Premier healthcare alliance, Malcolm Baldrige National Quality Award recipient
Premier is a performance improvement alliance of more than 2,700 U.S. hospitals and 90,000 other sites using the power of collaboration and technology to lead the transformation to coordinated, high-quality, cost-effective care. Owned by hospitals, health systems and other providers, Premier operates a leading healthcare purchasing network with more than $4 billion in annual savings. Premier also maintains the nation's largest clinical, financial and outcomes database with information on 1 in 4 patient discharges. A world leader in delivering measurable improvements in care, Premier works with the Centers for Medicare & Medicaid Services. Headquartered in Charlotte, N.C., Premier also has an office in Washington. http://www.premierinc.com. Stay connected with Premier on Facebook, Twitter and YouTube
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that SedLine facilitates more individualized titration of anesthesia that leads to patients receiving significantly less anesthetic and recovering faster, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that SedLine will facilitate immediate detection of asymmetrical activity in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Kennedy Health System Renews System-Wide Pulse Oximetry Contract with Masimo
Long-Standing Masimo SET® Pulse Oximetry Customer Now Leveraging Masimo rainbow® Pulse CO-Oximetry for Advanced Patient Monitoring
Irvine, California – October 31, 2012 – Kennedy Health System, encompassing three acute care New Jersey hospitals on the cutting edge of new technology and medical procedures, today renewed the health system's long-standing contract and commitment to Masimo SET® as its pulse oximetry standard-of-care. Kennedy Health System signed another five-year contract with Masimo for SET® pulse oximetry and added rainbow® Pulse CO-Oximetry for advanced, noninvasive patient monitoring.
Kennedy Health System standardized on Masimo SET® pulse oximetry technology for Measure-Through Motion and Low Perfusion SpO2, pulse rate, and perfusion index measurements more than 10 years ago. Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events.2 More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions – establishing the technology as an industry-leading pulse oximetry that substantially contributes to improved patient outcomes.
Today, the health system is expanding its relationship with Masimo and deploying two new Masimo rainbow® SET® Pulse CO-Oximetry measurements: noninvasive total hemoglobin (SpHb®) and Pleth Variability Index (PVI®). Masimo SpHb enables clinicians to measure hemoglobin without a painful needle stick or time-consuming blood lab analysis,3 while PVI helps clinicians to noninvasively and continuously assess the fluid status of patients without invasive procedures.4
Masimo rainbow® SET® technology has been shown to have the highest pulse oximetry specificity (lowest rate of false alarms) and sensitivity (highest true alarm detection),2 as well as noninvasive patient monitoring solutions that allow clinicians to measure multiple blood constituents – including: oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), acoustic respiration rate (RRa™), and perfusion index (PI) in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study.
3 Frasca D., Dahyot-Fizelier C., Catherine K., Levrat Q., Debaene B., Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit Patients." Crit Care Med. 2011 Oct;39(10):2277-82. http://www.masimo.com/pdf/clinical/hemoglobin/frasca-accuracy-of-a-continuous-noninvasive-hemoglobin-monitor-Crit-Care-Med-2011.pdf.
4 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot J.J. "Pleth Variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." Br J Anaesth. 2008 Aug;101(2):200-6. http://www.masimo.com/pdf/clinical/pvi/cannesson-pleth-variability-index-to-monitor-the-respiratory-variations-in-the-pulse-oximeter-plethysmographic-waveform-amplitude-and-predict-fluid-responsiveness-in-the-operating-theatre-aug-2008.pdf.
About Kennedy Health System
The Kennedy Health System is an integrated healthcare delivery system providing a full continuum of healthcare services, ranging from acute-care hospitals to a broad spectrum of outpatient and wellness programs. A multi-site healthcare provider, Kennedy serves the residents of Camden, Burlington and Gloucester counties. Our mission - to enhance the health status of the communities it serves - is further expanded through our educational commitment and affiliation with the University of Medicine and Dentistry of New Jersey. Kennedy provides the largest non-public physician training program in the state of New Jersey. Kennedy Health System has three hospital campuses in Southern New Jersey: Cherry Hill, Stratford and Washington Township. Please visit www.kennedyhealth.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb), PVI, and acoustic respiration rate (RRa™) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Masimo Reschedules Release of Third Quarter 2012 Financial Results and Associated Conference Call to Thursday, November 1st Due to Hurricane Sandy
Earnings release to be issued after market close Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 29, 2012 -- Masimo (NASDAQ: MASI) announced today that due to Hurricane Sandy it has rescheduled release of its third quarter 2012 financial results and associated conference call from Tuesday, October 30, 2012, to Thursday, November 1, 2012.
Masimo will release its financial results for the period ended September 29, 2012 after the market closes on November 1, 2012 and conduct a conference call to review the results at 1:30 p.m. PT (4:30 p.m. ET). The call will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
Masimo made the change to accommodate members of the investor and financial analyst community being impacted by possible sustained disruption from Hurricane Sandy.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 38439591. After the live webcast, the call will be available on Masimo's website through December 1, 2012. In addition, a telephonic replay of the call will be available through November 15, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 38439591.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

Alameda County Medical Center Deploys Masimo Patient SafetyNet™
Major California Hospital System Equipped with Masimo rainbow® Acoustic Monitoring for Ventilation Monitoring and Masimo SET® Pulse Oximetry for Oxygenation Monitoring
Oakland, California & Irvine, California – October 25, 2012 – Masimo (NASDAQ: MASI) and Alameda County Medical Center (ACMC) announced today the successful installation of Masimo Patient SafetyNet™ with rainbow® Acoustic Monitoring™ – advanced noninvasive technologies that help clinicians to save lives while helping hospitals to reduce costs.1
ACMC, a nationally recognized public healthcare system with more than 2,800 employees, 500 physicians, and 475 beds, is among a growing number of healthcare systems to standardize on Masimo SET® and the Masimo rainbow® SET® technology platform, including Patient SafetyNet, and rainbow® Acoustic Monitoring.
- Patient SafetyNet tracks the underlying physiological conditions of patients, and detects changes and abnormalities that signal declining health status in real time. When a patient's condition deteriorates, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
- rainbow® Acoustic Monitoring noninvasively and continuously measures a patient's respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck. Acoustic Respiration Rate (RRa™) is a breakthrough measurement that facilitates earlier detection of respiratory compromise and patient distress – especially important for post-surgical patients receiving patient-controlled analgesia for pain management, as the sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.2-4
"Masimo's Patient SafeyNet, coupled with Acoustic Monitoring, plays an important role in our efforts to provide the safest care possible to our patients after surgery," said Brandy Burrows, ACMC's Director of Respiratory Care Services. "Our goal is to provide the best care and Masimo's technology helps to safely meet recommendations from both the Joint Commission and the Anesthesia Patient Safety Foundation (APSF) to continuously monitor both oxygenation and ventilation of our post-surgical patients."5
Alameda County Medical Center has also converted its pulse oximetry technology to Masimo SET®, which has been shown to virtually eliminate false alarms6 and increase a clinician's ability to detect life-threatening events.7 More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions – helping to substantially contribute to improved patient outcomes. In addition, ACMC has also added Masimo rainbow® SET® Pulse CO-Oximetry capabilities—allowing clinicians to noninvasively monitor multiple blood constituents that previously required invasive or complicated procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa).
Michael O'Reilly, MD, Chief Medical Officer of Masimo, stated: "Patients and their families can be confident that with the Masimo Patient SafetyNet system, they are being monitored at the bedside even when clinical staff is not in the room. We are honored that Alameda County Medical Center has selected Masimo technology to help protect their patients and improve patient outcomes."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
2 Joint Commission on Accreditation of Healthcare Organizations. Sentinel event alert: patient controlled analgesia by proxy; JCAHO. 2004.
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part I – How errors occur; ISMP. 2003.
4 Bird M. Acute pain management: a new area of liability for anesthesiologists; ASA Newsletter. 2007; 71:8.
5 Stoelting RK, Weinger MB. Dangers of Postoperative Opioids -- Is There a Cure? APSF Newsletter, 2009;24:25-26. http://www.apsf.org/newsletters/html/2009/summer/index.htm
6Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
7 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
About Alameda County Medical Center
Alameda County Medical Center is a nationally recognized public healthcare system with more than 2,800 employees, 500 physicians, and 475 accredited beds. ACMC provides comprehensive, high quality medical treatment and compassionate care to all residents of Alameda County. The integrated system of hospitals, clinics, and health services is staffed by healthcare professionals who are responsive to the diverse cultural needs of our community. ACMC fosters a learning environment with a wide range of educational programs and activities including medical research and education for students, interns and residents as well as continuing medical education for medical, nursing and other staff. ACMC operates six facilities: Highland Hospital in Oakland, John George Psychiatric Pavilion in San Leandro, Fairmont Hospital in San Leandro and three community-based wellness centers. The system provides a range of services from family and women's health to pediatrics, oncology and orthopedics. For more information, please visit www.acmedctr.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, and acoustic respiration rate (RRa™) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jerri Applegate Randrup
Alameda County Medical Center
(510) 437-4732
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Clinical Study Finds Pronto® Has Similar Accuracy to Commonly Used Point-of-Care Invasive Hemoglobin Analyzer
Irvine, California – October 24, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in Postgraduate Medicine demonstrates noninvasive total hemoglobin (SpHb®) measurements from the Pronto have an accuracy similar to those of the most commonly used invasive point-of-care (POC) hematology analyzer (HemoCue® 201+, Quest Diagnostics) in outpatient settings.1
Total hemoglobin (Hb) is one of the most frequently ordered laboratory tests in hospitals, urgent care centers, physician offices, and public health clinics.2 Anemia, defined as low hemoglobin concentration, is among the most common blood disorders in the United States, afflicting an estimated 3.4 million Americans, according to the National Center for Health Statistics. Many people are at risk for anemia because of poor diet, intestinal disorders, chronic diseases, infections, and other conditions. The signs and symptoms of anemia can be easily overlooked, and many people do not realize they have anemia until it is identified by a blood test.
Yet conventional invasive POC testing is time-consuming and exposes the patient and clinician to potential contamination and exposure to blood-borne pathogens. Errors occurring during the pre-analytical phase – from the time the test is ordered by the physician until the sample is ready for analysis – can account for up to 93% of the errors currently encountered during the total diagnostic process.3 A review of multiple studies in 2002 showed similarly high levels of errors.
The study that appears in Postgraduate Medicine, by Marina Raikhel, M.D., of the Torrance Clinical Research Institute in Lomita, Calif., measured hemoglobin of 152 subjects (69% women) presenting at an outpatient research clinic with common conditions such as diabetes, asthma, high cholesterol and high blood pressure.
Compared to laboratory analysis of blood, the bias ± standard deviation were −0.5 ± 1.0 g/dL for SpHb and 0.3 ± 1.0 g/dL for HemoCue 201+. Noninvasive SpHb testing had bias and standard deviation similar to those of HemoCue 201+, according to the study.
| Device | Pronto | HemoCue |
|---|---|---|
|
Sample Type |
Noninvasive |
Invasive |
|
Methodology |
Spectrophotometric |
Photometric |
|
Bias |
-0.5 g/dL |
0.3 g/dL |
|
SD |
1.0 g/dL |
1.0 g/dL |
|
Limit of agreement |
-2.5 to 1.5 g/dL |
- 1.7 to 2.3 g/dL |
"Because SpHb measurement is noninvasive, it has the potential to confer the additional benefits of patient comfort, decreased complexity, and increased safety for health care providers who are not exposed to risks of needle-stick injury and blood-spill contaminations," the study concluded.
"As this study shows, the accuracy of the Pronto provides clinicians a powerful, yet painless, tool to test total hemoglobin without many of the risks associated with conventional, invasive testing," said Dr. Michael O'Reilly, Chief Medical Officer at Masimo. "The simplicity, safety and accuracy of the Pronto can help hospitals, clinics and physician offices improve processes and patient outcomes."
1 Raikhel M. "Accuracy of Noninvasive and Invasive Point-of-Care Total Blood Hemoglobin Measurement in an Outpatient Setting" Postgraduate Medicine, Volume 124, Issue 4, July 2012, ISSN – 0032-5481, e-ISSN – 1941-9260.
2 Steindel SJ, Rauch WJ, Simon MK, Handsfield J. National Inventory of Clinical Laboratory Testing Services (NICLTS). Development and test distribution for 1996. Arch Pathol Lab Med. 2000;124:1201-1208.
3 Preanalytical variability: the dark side of the moon in laboratory testing. G Lippi, GC Guidi, C Mattiuzzi, and M Plebani. Clin Chem Lab Med, Jan 2006; 44(4): 358-65.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Multiple New Clinical Studies Presented at the American Society of Anesthesiologists Annual Meeting Show Benefits of Masimo Noninvasive Patient Monitoring Technologies: SpHb, PVI, RRa, and SEDLine
Irvine, California – October 18, 2012 – Masimo (NASDAQ: MASI) announced today that eight significant new clinical studies evaluating Masimo noninvasive patient monitoring technologies were presented at the largest gathering of anesthesiologists in the world, the American Society of Anesthesiologists (ASA) Annual Meeting in Washington, D.C. The following studies highlight the positive clinical outcomes and patient safety impact of Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® brain function monitoring.
Noninvasive Total Hemoglobin (SpHb®)
At Fujisawa Municipal Hospital, in Fujisawa, Japan, researchers found that "continuous monitoring of SpHb by pulse oximetry enabled us to evaluate relative change in blood volume and adjust ultrafiltration rate to plasma refilling rate," and concluded: "The results of this study indicate that SpHb monitoring contributes to effective ultrafiltration and stable blood purification."1
At the Seoul National University Hospital, in Seoul, Korea, researchers found that the change in bias and precision of SpHb when compared to invasive laboratory hemoglobin was not significantly different before vs. after intravenous volume loading in children during neurosurgery. The study concluded: "The Radical-7 Pulse CO-Oximeter® can be useful as a trend monitor in children during operation even immediately after volume expanders are administered."2
In another study, researchers Nitin Shah, M.D., and Deval Modi, M.B.,B.S., at the VA Long Beach Healthcare System in Long Beach, Calif., conducted noninvasive hemoglobin testing in patients undergoing cataract surgery in South Africa and with health fair participants in Southern California and showed that Masimo's Pronto-7® noninvasive spot-check hemoglobin device performs "with acceptable reliability" in populations with darker skin pigmentation and "was able to obtain SpHb readings in the vast majority of subjects of both light and dark skin pigmentation."3
Pleth Variability Index (PVI®)
A study at the University of Bordeaux, in Bordeaux, France, by researchers Thakoor Bhismadev, M.D., Tarik Riahi, M.D., Olivier Bernard, M.D., Musa Sesay, M.D., Pierre Maurette, M.D., and Karine Nouette-Gaulain, M.D., , found that "PVI can reliably predict fluid responsiveness in neurosurgical patients in the ICU."4
rainbow® Acoustic Monitoring™ for RRa™
In a multi-center study at Cincinnati Children's Hospital Medical Center, University of Arizona Medical Center, and Children's Medical Center at Dallas, by researchers Mario Patino, M.D., Mohamed Mahmoud, M.D., Dean Kurth, M.D., Daniel Redford, M.D., Thomas Quigley, M.D., and Peter Szmuk, M.D., found that RRa "showed a bias, precision and accuracy root mean square (Arms) of -0.02, 3.25 and 3.25 breaths per minute (bpm) compared to the reference method, whereas capnography showed a bias, precision and Arms of 0.23, 3.29 and 3.30 bpm." They concluded: "This multicenter study showed that respiratory rate measured from noninvasive, acoustic monitoring had similar accuracy and precision as nasal capnography, the current standard of care when used in pediatric patients."5
A study at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, by Jeff Mandel, M.D., M.S., and Joshua Atkins, M.D.,Ph.D., found that RRa compared favorably to capnography and respiratory inductance plethysmography (RIP), concluding that RRa "displayed good accuracy and precision compared to capnography, demonstrating a good alternative for patients not tolerating capnography." Researchers noted that RRa comparison to RIP produced a larger bias and precision, possibly due to noise of RIP recordings during patient transport from OR to PACU, showing RRa utility under such interfering conditions for RIP."6
A separate study at Stanford University School of Medicine, by Pedro Tanaka, M.D., David Drover, M.D., and Maria Tanaka, M.D., evaluated the accuracy of respiration rate monitored by CapnoStream (RRetCO2) and RRa in anesthetized patients under sedation and concluded: "The use of RRa could be a good alternative for the perioperative period as most patients will have received sedation during surgery and need to be monitored in PACU and later on the patient wards."7
SEDLine®
Results from a study conducted at the University of California San Francisco by researchers Susana Vacas, M.D., Erin McInrue, M.P.H., Mervyn Maze, M.D., and Jacqueline Leung, M.D.,Ph.D., using the SEDLine 4-channel brain function monitor "confirmed the utility of a portable monitor to measure different sleep stages." Researchers concluded: "Having the ability to continuously monitor sleep in the ICU setting will facilitate clinical trials with goal-directed interventions that rectify sleep disruption. Targeting modifiable risk factors such as sleep disruption may ultimately decrease delirium and associated adverse events in the critically ill patients."8
1 Yamada H, Minako S, Sakamoto S, Junko I, Saitoh M, Minoguchi K, Kawada K, Higurashi A, Takahashi S, Takeda K. "Measurement of Relative Change in Blood Volume and Plasma Refilling Rate During Hemodialysis by Noninvasive Continuous Hemoglobin Monitoring Using Pulse Oximetry" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. 8A811.
2 Park Y, Kim J-T, Byon H, Kim H-S. "The Accuracy of Noninvasive Hemoglobin Monitoring Using Radical-7 Pulse CO-Oximeter in Children Undergoing Neurosurgery" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. A421.
3 Shah N, Modi D. "Performance of Pronto-7 Noninvasive Hemoglobin Pulse CO-Oximeter in a Dark Skinned Population" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. A573.
4 Bhismadev T, Riahi T, Bernard O, Sesay M, Maurette P, Nouette-Gaulain K. "Comparison Between Pleth Variability Index and Arterial Pulse Pressure Variation in the Neurosurgical Intensive Care Unit (ICU)" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. A908.
5 Patino M, Mahmoud M, Kurth D, Redford D, Quigley T, Szmuk P. "Accuracy of Acoustic Respiration Rate Monitoring in Pediatric Patients" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. JS10.
6 Mandel J, Atkins J. "Reliability of Masimo Rainbow Acoustic Monitoring in Patients Undergoing Elective Procedures Under General Anesthesia" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. A093.
7 Tanaka P, Drover D, Tanaka M. "Correlation Between RRetCO2 and Masimo RRA in Patients Under Deep Sedation: A Pilot Assessment" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. A571.
8 Vacas S, McInrue E, Maze M, Leung J. "Sleep Disruption in the Intensive Care Unit" Proceedings of the American Society of Anesthesiologists, October 13, 2012. Washington DC. A396.
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: www.masimo.com/home/clinical-evidence/clinical-evidence.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report Third Quarter 2012 Financial Results after Market Close on October 30 Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 16, 2012 -- Masimo (NASDAQ: MASI) announced today that it will release third quarter financial results for the period ended September 29, 2012, after the market closes on October 30, 2012. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 38439591. After the live webcast, the call will be available on Masimo's website through November 30, 2012. In addition, a telephonic replay of the call will be available through November 13, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 38439591.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Initiates Limited Market Release of Universal ReSposable™ Pulse Oximetry Sensor System
Offering a Green, Measure-Through Motion and Low Perfusion Performance Alternative
Irvine, California – October 15, 2012 – Masimo (NASDAQ: MASI) announced today limited market release of the Universal ReSposable™ Pulse Oximetry Sensor System, offering the Measure-Through Motion and Low Perfusion performance and accuracy of SET®, as well as the comfort of its single-patient-use adhesive sensors with the cost-effectiveness and environmental advantages of a reusable sensor.
The new Universal ReSposable pulse oximetry sensor was created after more than 10 years of research and development with feedback from hundreds of clinicians on what they wanted most in a sensor – less waste, more value, and the Masimo SET® performance.
The new ReSposable sensor components sit atop the sensor – not embedded – reducing risk of pressure points. Patients also can be disconnected from a pulse oximeter without having to carry the sensor cable.
"We've been impressed not only with the reduction in costs, but the ReSposable sensor is helping us meet our environmental, waste-reduction goals," said SuzAnne J Gil, Clinical Resource Manager at San Juan Regional Medical Center in Farmington, New Mexico. "In an era when hospitals are expected to deliver quality care, while controlling costs and carbon footprints, Masimo's ReSposable sensors offer a clear benefit for patients, clinicians and facility administrators."
"The Masimo innovative ReSposable concept should be considered for any clinical unit regarding SpO2 sensor usage because of its sustainability both in terms of cost-control and waste-reduction policies," said Dr. Concha Goñi, head of neonatal and pediatric intensive care units at Hospital Virgen del Camino in Pamplona, Spain, where the sensor has been used as part of Masimo's pre-market release testing.
Masimo's Universal ReSposable SpO2 sensors generate up to 90% less waste vs. 34% less waste with reprocessing,* while reducing carbon emissions up to 41% vs. 43% more emissions with reprocessing.**
Disposable pulse oximetry sensors historically have offered the best performance, greatest ease of use, and most comfort, but do generate more waste than reusable sensors. Reusable sensors may offer low environmental impact, but do not offer the same performance, comfort, or reduction of cross-contamination risk as disposable sensors. And third-party reprocessed sensors do not offer the same performance or quality as new sensors, as evidenced by their own indications for use statements. Moreover, reprocessed sensors have a higher carbon footprint than new sensors, due to greater greenhouse gas emissions from transport, reprocessing, and decontamination procedures.
Joe Kiani, CEO and Founder of Masimo, said, "More than ever, hospitals are under pressure to reduce costs and implement green initiatives, while maintaining infection-control practices to protect patients and staff from cross-contamination. While the ultimate way to reduce cost and improve care is through process of care improvements made possible by technologies like SET® and rainbow®, with ReSposable sensors, hospital materials management departments can meet the cost reduction edicts, while helping their hospitals meet their environmental, and safety goals, and most importantly ensure that their patients are provided with accurate monitoring when they need it most."
Contact your local Masimo sales representative for demonstration of the Universal ReSposable Sensors. To locate a Masimo representative or distributor in your area and evaluate the Universal ReSposable SpO2 Sensor System, call 1-800-257-3810 or visit www.masimo.com.
*Waste calculated by sensor weight for 40% reprocessed sensors with a mix of 80% Adult and Pediatric sensors, 20% Neo and infant sensors.
**Carbon footprint comparisons calculated by lbs. CO2 emissions with same reprocess mix as waste. Carbon footprint calculations validated by Carbonfund.org in November, 2011.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms all other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow SET® technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the Universal ReSposable SpO2 Sensor System, the comfort of the single-patient-use adhesive sensor, its cost-effectiveness, and environmental advantages; and our assumptions that rainbow Universal SuperSensor enables continuous measurement of SpHb, SpCO, SpMet, SpO2 and the new SpfO2 parameter that allows precise oxygen saturation even in the presence of dyshemoglobins, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Unveils Fractional Arterial Oxygen Saturation, SpfO2, and rainbow SuperSensor
SpfO2 Enables More Accurate Oxygenation Assessment in the Presence of Dyshemoglobins
Irvine, California – October 12, 2012 – Masimo (NASDAQ: MASI) today announced the CE Mark and debut of the new fractional arterial oxygen saturation, SpfO2™, parameter through the rainbow® Universal ReSposable SuperSensor™, the first noninvasive sensor to provide simultaneous monitoring of SpHb®, SpCO®, SpMet®, SpfO2, SpOC™, Perfusion Index, PVI®, and Measure-Through Motion and Low Perfusion SpO2 and pulse rate.
-
CAPTION: The rainbow® Universal ReSposable SuperSensor™ – with disposable optical sensor (Super UR-DOS™ – top) and reusable optical sensor (Super UR-ROS™) – noninvasively measures oxygenation in the presence of dyshemoglobins, which may allow for better therapeutic decisions.
Masimo will debut the SpfO2 and the rainbow® SuperSensor at the American Society of Anesthesiologists' 2012 Annual Meeting in Washington, D.C., tomorrow.
Until now, pulse oximeters could only measure and display functional oxygen saturation (SpO2). So, when patients had elevated carboxyhemoglobin (from carbon monoxide poisoning) and/or elevated methemoglobin (negative reaction to more than 30 common drugs used in hospitals, like caines, nitrates, and Dapsone), the displayed functional oxygen saturation overestimated the actual oxygen saturation value.
Utilizing more than seven wavelengths of light and breakthrough signal processing, Masimo rainbow® Pulse CO-Oximeters™ can measure and display fractional arterial oxygen saturation (SpfO2). SpfO2 allows more precise arterial oxygenation assessment in patients with elevated dyshemoglobins, common throughout the hospital and pre-hospital setting, compared to functional oxygen saturation (SpO2).
SpfO2 may allow earlier interventions and more timely therapeutic decisions. For example, in a patient who is a smoker with an SpO2 of 98%, carboxyhemoglobin level of 12% and methemoglobin of 1%, if SpfO2 were available, it would be displayed at 85%. It is well accepted that clinicians would frequently make different diagnostic and therapeutic decisions at an oxygenation of 85% versus 98%.
"The new fractional arterial oxygenation measurement adds to the unprecedented noninvasive monitoring innovations Masimo has brought to clinicians, providing greater insight into the true state of their patients," said Masimo CEO and founder Joe Kiani. "SpfO2 will also be used with the noninvasive and continuous hemoglobin (SpHb) measurement, to assess the patient's true arterial oxygen content (SpOC) and should lead to better decisions about common therapies such as administering oxygen or red blood cell transfusions."
Both SpfO2 and the rainbow® SuperSensor are pending FDA 510(k) clearance.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the FDA clearance, timing and availability of rainbow Universal ReSposable Super Sensors and SpfO2 parameter; that SpfO2 and SuperSensors represent an innovative solution to more accurately measure blood oxygenation and hemoglobin, which could allow clinicians to make earlier interventions or better therapeutic decisions, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

GE Healthcare Integrates New FDA-Cleared Masimo SET® uSpO2™ Pulse Oximetry Cable into GE's ApexPro® Telemetry Systems
Irvine, California – October 9, 2012 – Masimo (NASDAQ: MASI) today announced the FDA 510(k) clearance and market release of Masimo's new uSpO2™ universal pulse oximetry cable for use with GE Healthcare's ApexPro® Telemetry Systems. GE Healthcare is the first to integrate Masimo's low power platform with SET® Measure-Through Motion and Low Perfusion SpO2technology into patient-worn monitors designed for use in ambulatory patient care environments.
Ambulatory care environments such as telemetry and cardiac step-down units often use patient-worn monitors to conduct continuous monitoring during patient ambulation. In addition to the motion artifact caused by this ambulation, cardiac patients can experience low peripheral perfusion on an intermittent or consistent basis. However, these monitoring conditions have proven to be particularly challenging for conventional pulse oximetry technology and may lead to inaccurate pulse oximetry measurements and nuisance alarms that detract from patient care.1 As demonstrated in more than 100 independent and objective studies, Masimo SET® pulse oximetry is clinically proven to virtually eliminate false alarms1 and increase a clinician's ability to detect life-threatening events2 under challenging conditions including patient motion and low peripheral perfusion.

-
CAPTION: Masimo's new FDA-Cleared uSpO2™ cable (right) provides plug-and-play Masimo SET® pulse oximetry functionality with GE's ApexPro® Telemetry Systems (left).
Masimo's uSpO2™ (pictured) is a "Board-in-Cable" external pulse oximetry solution that provides a simple way to add Masimo SET® pulse oximetry for devices that are not designed to have SpO2integrated inside the device. The uSpO2™ cable embeds a Masimo MS-2040 circuit board within a completely self-contained cable that attaches to the patient sensor.
The uSpO2™ cable adapted for the GE ApexPro® Telemetry Systems provides full Masimo SET® pulse oximetry capabilities with proven accuracy during patient motion and low perfusion, while reducing Masimo's power consumption to approximately 60 milliwatts, which reflects a 97% reduction to initial SET® SpO2platforms and a 52% reduction vs current SET® platforms.
Masimo's new uSpO2™ cable has been adapted to provide plug-and-play functionality with GE's ApexPro® Telemetry Systems. As a result, clinicians can upgrade their monitoring capabilities by purchasing Masimo's uSpO2™ cable for their GE ApexPro Telemetry System – without the cost of additional hardware or software. And for the hundreds of GE customers who have standardized on Masimo SET® SpO2technology within their GE bedside monitors, the uSpO2™ cable enables standardization of SpO2technology and sensors as patients transition between care areas—from bedside monitors in hospital rooms to patient-worn transmitters in ambulatory care areas. This measurement technology and sensor standardization can help healthcare providers reduce costs, while enhancing workflow, care processes, and efficiencies.
Today's announcement builds on a long-term existing partnership between GE Healthcare and Masimo. Earlier this year, GE Healthcare and Masimo jointly announced an expanded long-term agreement under which GE Healthcare would incorporate Masimo rainbow® SET® technology into many of GE Healthcare's patient monitoring products.
Rick Fishel, Masimo President of Worldwide OEM Business and Corporate Development, stated, "Masimo is focused on delivering innovative technology solutions that allow caregivers to better address the needs of their patients under the most challenging environments. We are pleased that GE Healthcare is the first patient monitoring company to combine Masimo's low power SET® technologies in ambulatory patient applications, further enhancing GE's comprehensive wireless solutions that enable caregivers to work more productively and respond to critical situations faster. We believe this new level of functionality in GE's patient-wearable monitors capitalizes on our technology partnership and reflects our shared corporate visions to improve patient outcomes while reducing the cost of healthcare delivery."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms all other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow SET® technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the reduced power consumption profile stated for the uSpO2for GE ApexPro will be consistent for other telemetry systems; that the uSpO2delivers a new level of functionality in GE's patient-wearable monitors that will help to improve patient outcomes while reducing the cost of healthcare delivery; and that uSpO2will facilitate measurement technology and sensor standardization that can help healthcare providers reduce costs, while enhancing workflow, care processes, and efficiencies; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Albany Medical Center Standardizes to Masimo rainbow SET Pulse CO-Oximetry®
Leading-Edge Northeastern New York Hospital Leverages Advanced Medical Technologies
for Noninvasive, Continuous Patient Monitoring
Livewire – Irvine, California – October 2, 2012 – Masimo (NASDAQ: MASI) today announced the conversion of Albany Medical Center (AMC), one of the biggest hospitals in the Capital (N.Y.) Region, to Masimo rainbow SET Pulse CO-Oximetry and sensor technologies for advanced noninvasive patient monitoring capabilities.
The conversion equips AMC – which incorporates the 651-bed Albany Medical Center Hospital and the Albany Medical College – with breakthrough rainbow® technology that allows clinicians to measure multiple blood constituents, fluid responsiveness, respiration rate, and other physiological parameters without invasive blood draws and time-consuming laboratory analysis. Parameters in use include total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The pulse oximetry standard-of-care at leading hospitals worldwide, Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events.2
"The ability to monitor surgical patients' hemoglobin noninvasively and continuously with Masimo technology helps us better manage blood use in the ICU," said Heidi DeBlock, MD, FCCM, and Associate Professor of Surgery Albany Medical Center. "More timely measurement of a patient's hemoglobin allows us to potentially reduce costly blood transfusions, which can be risky and unnecessary. Not only does Masimo's technology help save patients, it helps save money."
Albany Medical Center Hospital provides care to more than 32,500 inpatients and 533,000 outpatients annually. The Albany Medical College, meanwhile, trains the next generation of doctors, scientists and other healthcare professionals and is home to the region's largest physicians practice with 325 doctors.
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
About Albany Medical Center
Albany Medical Center is northeastern New York's only academic health sciences center and is one of the largest private employers in the Capital Region. It incorporates the 651-bed Albany Medical Center Hospital, which provides care to more than 32,500 inpatients and 533,000 outpatients annually and offers the widest range of medical and surgical services in the region, and the Albany Medical College, which trains the next generation of doctors, scientists and other healthcare professionals and is home to the region's largest physicians practice with 325 doctors. For more information visit www.amc.edu
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all AMC patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Hamilton Medical Center Updates with Masimo Patient Monitoring Technologies
Dalton, Georgia & Irvine, California – October 1, 2012 – Masimo (NASDAQ: MASI) and Hamilton Medical Center in Dalton, Ga., announced today the hospital has updated to Masimo SET® pulse oximetry and rainbow® Pulse CO-Oximetry, offering clinicians demonstrably advanced noninvasive and continuous patient monitoring designed to improve patient outcomes while helping hospitals reduce costs.
"Hamilton Medical Center prides itself on offering some of the most advanced medical technology available," said Jeff Hughes, Hamilton's Director of Cardiopulmonary and Sleep Services. "We use Masimo because we found that its innovative patient-monitoring solutions outperform competing devices, particularly in challenging patient-care environments."
Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events.2 Masimo rainbow® SET® allows clinicians to measure multiple blood constituents and other physiological parameters without time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
Hamilton Medical Center also has installed Masimo Patient SafetyNet™, clinically shown to reduce rapid response activations, intensive care unit (ICU) transfers, and deaths related to opioid-induced respiratory depression, while offering significant opportunity cost savings.3 When a monitored patient's condition deteriorates, the Patient SafetyNet system alerts clinicians – prompting a potentially lifesaving response at the patient's bedside.
For the last two years, HealthGrades has named Hamilton Medical Center a Distinguished Hospital for Clinical Excellence – a prestigious distinction that places Hamilton Medical Center among the top 5% of hospitals nationwide for clinical performance.
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.jcafulltextonline.com/article/S0952-8180(12)00148-1/abstract
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Studyne
3 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
About Hamilton Medical Center
Hamilton Medical Center is a 282-bed regional acute-care hospital that offers major medical, surgical and diagnostic services, including accredited stroke and chest pain centers. Included under Hamilton Medical Center are the Bradley Wellness Center, Hamilton Specialty Care, Hamilton Home Health and Hamilton Hospice. Visit www.hamiltonhealth.com
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Masimo Announces $1 Million Commitment to Action with the Clinton Global Initiative
to Solve Maternal Mortality & Anemia
Two-Year Project Will Increase Access to Anemia Screening, Intervention and Education
Irvine, California – September 25, 2012 – Masimo (NASDAQ: MASI) announces first-ever Commitment to Action with the Clinton Global Initiative (CGI) to solve the global problem of maternal mortality and anemia—beginning with Liberia and Uganda. The $1 million, two-year project initially focuses on five villages in Liberia and Uganda—two countries where the epidemics of maternal mortality and anemia are among the worst. Masimo is also working to formalize new partnerships that will expand the commitment to additional countries and communities in an effort to combat maternal mortality and anemia in resource-poor countries across the globe.
According to a joint statement by the World Health Organization (WHO) and UNICEF, "anemia is a widespread public health problem with major consequences for human health as well as social and economic development."1 The most dramatic health effects of anemia—maternal and child mortality—mean that mothers and young children in developing countries face double jeopardy from preventable causes.
Anemia: An estimated two billion people worldwide are anemic—this represents one quarter of the world's population, with women and children under five representing the highest risk groups for chronic anemia. In fact, pregnancy-related anemia is a common condition. Severe or long-lasting anemia can result in cognitive, neurological and cardiac damage, or death.1
Maternal Mortality: Every 90 seconds, a woman dies from complications related to pregnancy or childbirth in the developing world. One of every three maternal deaths (in Africa and Asia) is attributed to significant bleeding, called post-partum hemorrhage (PPH).1
Anemia and PPH (excessive bleeding during childbirth) can be treated and prevented if identified early; however, hemoglobin blood tests used to diagnose both anemia and excessive bleeding typically require an invasive blood draw and laboratory analysis. This is always painful and if done inappropriately may cause infection and bleeding. Moreover, trained persons, equipment and supplies required to complete the blood draw and lab analysis are often not available or too expensive for resource-poor countries and rural areas. Today, there is an easy and cost-effective way to measure hemoglobin in any setting. Masimo is the first and only company to have a commercially available noninvasive hemoglobin monitor with FDA clearance. Thanks to Masimo, its handheld hemoglobin monitor, called the Pronto™, can noninvasively measure hemoglobin levels in under a minute. By placing a noninvasive finger sensor on the patient and pushing a button, the device can quickly measure if a mother or child is suffering from low hemoglobin.
The public-private partnership commitment includes: a funding grant of $300,000 provided by the Masimo Foundation for Ethics, Innovation, and Competition in Healthcare; in-kind equipment donation of $550,000 in oximeters and sensors provided by the Masimo Corporation, and additional support from Tiyatien Health—a nonprofit organization that saves lives in remote villages of Liberia by giving war survivors, gifted women, and former patients the training, equipment and support they need to become frontline health workers. In addition, the resources, operational systems and anemia management experts of the Opio Uganda Team (OUT) will facilitate the outreach efforts in Uganda.
Officially launching in October 2012, the Maternal Mortality and Anemia project will consist of four phases:
Phase I: Mobilize Resources & Implement Routine Anemia Screening, Education and Supplementation
Phase II: Measurement of Sustainability of Screening
Phase III: Measurement of Compliance to Education and Supplementation
Phase IV: Identification of Health and Wellness Impact and Long-term Sustainability
Masimo Founder and CEO, Joe Kiani, who made the project announcement at the CGI Annual Meeting, stated, "Solving unsolvable health problems is in our DNA. We recognize our responsibility and the important application of our noninvasive hemoglobin technology and devices as a solution to address the global challenge of anemia and maternal death. Our $1 million Commitment to Action with the Clinton Global Initiative and collaboration with Tiyatien Health (TH) in Liberia and the Opio Uganda Team (OUT) will provide the devices, supplies, and mobilize the necessary ground resources to help increase access to anemia screening, intervention and education in these countries. This year, CGI's theme 'designed for impact' is particularly impactful because the Pronto™, with its breakthrough capability, ruggedness and cost point, as well as our plan of arming and training frontline health workers to diagnose and treat anemia and excessive maternal bleeding are both truly well-designed to make huge impact."
Dr. Raj Panjabi, Associate Physician with the Division of Global Health Equity at Brigham and Women's Hospital, Harvard Medical School, and Co-founder of Tiyatien Health (TH) in Liberia, says: "We at Tiyatien Health share in Masimo's vision that frontline health workers and the rural mothers they serve deserve equal access as everyone else in the world to Pronto™ and other tools of modern medicine. Advancing this human dignity is why TH is most proud to partner with Joe Kiani and the Masimo Foundation, and why their commitment will matter for rural mothers around the world."
According to Dr. Christopher K. Opio, an Ugandan internal medicine and digestive diseases physician with the Opio Uganda Team (OUT): "As physicians, we are always looking for more efficient means of diagnosing and treating our patients. Innovations like point-of-care testing of hemoglobin meet this need by providing an efficient, cost effective, and practical solution to problems related to hemoglobin estimation in resource limited settings."
Pronto™ also provides the Measure-Through Motion and Low Perfusion performance of Masimo SET® pulse oximetry with the highest sensitivity and specificity2 and proven ability to measure through a variety of skin pigments—even opaque. This makes Pronto™ even more impactful in helping health workers in rural villages throughout Africa.
1 "Focusing on Anemia: Towards an Integrated Approach for Effective Anemia Control" Joint Statement by the World Health Organization and the United Nations Children's Fund; WHO 2004. http://whqlibdoc.who.int/hq/2004/anaemiastatement.pdf.
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
About Clinton Global Initiative
Established in 2005 by President Bill Clinton, the Clinton Global Initiative (CGI) convenes global leaders to create and implement innovative solutions to the world's most pressing challenges. CGI Annual Meetings have brought together more than 150 heads of state, 20 Nobel Prize laureates, and hundreds of leading CEOs, heads of foundations and NGOs, major philanthropists, and members of the media. To date CGI members have made more than 2,100 commitments, which are already improving the lives of nearly 400 million people in more than 180 countries. When fully funded and implemented, these commitments will be valued at $69.2 billion.
CGI also convenes CGI America, a meeting focused on collaborative solutions to economic recovery in the United States, and CGI University (CGI U), which brings together undergraduate and graduate students to address pressing challenges in their community or around the world. For more information, visit clintonglobalinitiative.org and follow us on Twitter @ClintonGlobal and Facebook at facebook.com/clintonglobalinitiative.
About the Masimo Foundation for Ethics, Innovation and Competition in Healthcare
Committed to advancing positive change for the benefit of patients, clinicians, hospitals and payers everywhere. the Masimo Foundation for Ethics, Innovation, and Competition in Healthcare is a U.S.-based, private charitable foundation that is focused on improving patient care, preserving patient dignity and reducing cost of care, through philanthropic programs and research initiatives that foster an environment of aligned incentives, highest level of ethics for those who take part in the care of patients, and healthy and honest competition. The Masimo Foundation also helps create pathways for new lifesaving discoveries and inventions, and improve access to cost-effective, innovative healthcare solutions. The Foundation's emphasis is on transformative projects that seek to truly enhance patient safety and outcomes; helping to forge a world free of sickness, disease and inhumanity. Masimo Foundation supports third-party research, development initiatives, and clinical studies designed to expand the healthcare industry's ability to provide better and more cost-effective solutions and protocols for healthcare delivery. The Foundation also gives special attention to causes whose goals are ethical - focused on doing the right things for the right reasons - and designed to foster innovation and create healthy competition, which the Foundation believes is the ultimate answer to improving care, improving access to care, and lowering healthcare costs in the United States and around the world.To learn more about the Masimo Foundation, visit www.masimofoundation.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Clinton Global Initiative
Press Office
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Oximeters and Neonatal Sensors Receive FDA 510(k) Clearance with Labeling for Use in Newborn Screening for Critical Congenital Heart Disease (CCHD)
Masimo Announces HEART Program for CCHD Screening to Provide a Free Masimo SET® Pulse Oximeter to Hospitals in Need
Irvine, California – September 24, 2012 – Masimo (NASDAQ: MASI) announced today that it has received U.S. FDA 510(k) clearance for Masimo Signal Extraction Technology® (SET®) pulse oximeters, rainbow® SET® Pulse CO-Oximeters®, and neonatal sensors with labeling for screening newborns for critical congenital heart disease (CCHD). Masimo SET® pulse oximeters and sensors have previously been cleared to measure oxygen saturation and pulse rate during motion and low perfusion conditions in newborns, but this marks the first time the FDA has cleared specific labeling indicating the use of pulse oximeters, in conjunction with a physical exam, to screen newborns for CCHD.
In conjunction with the FDA clearance, Masimo also announced the HEART Program (Help Ensure Access to the Right Technology) for CCHD screening enabling hospitals in countries where Masimo has a presence that want to perform CCHD screening with a Masimo SET® pulse oximeter, but do not have one and do not have funds to purchase one, to receive a free Masimo SET® pulse oximeter. More details are available at www.masimo.com/home/signal-extraction-pulse-oximetry/technology-overview/clinical-studies.
CCHD causes up to 3% of all infant deaths in the first year of life.1 According to the U.S. Department of Health and Human Services (HHS), these types of heart defects affect about 7 to 9 of every 1,000 live births, one quarter of which could be detected and potentially treated by measuring blood oxygen saturation. FDA clearance comes as California recently became the latest state to mandate CCHD pulse oximetry screening (http://www.aroundthecapitol.com/Bills/AB_1731/20112012/), following HHS's September 2011 action to add pulse oximetry CCHD screening for newborns as part of the Recommended Uniform Screening Panel.
HHS took this action based on the published findings of the CCHD Workgroup,2 which relied on two major independent, published, prospective clinical studies that exclusively used Masimo SET® Measure-Through Motion and Low Perfusion pulse oximeters to recommend screening with "motion-tolerant pulse oximeters" that "have been validated in low perfusion conditions." Both of the studies were submitted by Masimo to the FDA to support the new CCHD screening labeling.
- Dr. Anne de-Wahl Granelli, et al., reported on the results of screening 39,821 newborn subjects at five maternity centers in Sweden. Investigators used the Masimo Radical™ with Masimo SET® technology, and found pulse oximetry screening of all well babies in maternity units is practically feasible with a minimum use of nursing time, and that it significantly improves detection of duct dependent heart disease before hospital discharge. The low false positive rate, the fact that other important pathology is unearthed by the screening, and the likely reduced need for preoperative neonatal intensive care suggest that such screening will be cost effective.3
- Dr. Andrew Ewer, et al., studied 20,055 newborn subjects at six maternity centers in the UK. Investigators used the Radical-7® with Masimo rainbow® SET® technology and found pulse oximetry to be a safe, feasible test that adds value to existing screening. It identifies cases of critical congenital heart defects that go undetected with antenatal ultrasonography, and the early detection of other diseases is an additional advantage.4
In spite of the wide availability of Masimo SET® pulse oximetry, both as standalone products as well as integrated products in over 100 multiparameter monitors from over 50 brands, some hospitals still do not have Masimo SET® technology. Given the strong evidence supporting the use of Masimo SET pulse oximetry for CCHD screening and Masimo's commitment to help save and improve babies' lives through expanded CCHD screening programs, the company's HEART Program (Help Ensure Access to the Right Technology) for CCHD screening will help ensure that hospitals have access to Masimo SET technology for CCHD screening.
"We are very proud of where we have taken pulse oximetry," stated Masimo founder and CEO, Joe Kiani. "Before SET pulse oximetry, CCHD screening was impractical, if not impossible with pulse oximetry. Dr. Graneli's initial study showed that even a so called 'next generation' pulse oximeter wasn't able to reliably work for CCHD screening, and was abandoned in the middle of the trial, while Masimo SET delivered the groundbreaking results of high sensitivity and specificity that became the basis for this new standard of care. We are truly elated with this new FDA clearance. We feel that with it, comes the responsibility to make CCHD pulse oximetry screening more accessible for infants, the most defenseless patients in any healthcare setting. We are excited to announce that, in conjunction with this new FDA clearance, we are launching the HEART Program for CCHD screening—offering a free Masimo SET® pulse oximeter to hospitals that need one but can't afford it. In this way, we are helping to address unmet needs on behalf of newborns around the world."
The Masimo pulse oximeters that were the subject of this 510(k) clearance are the Radical-7®, Rad-57™ and Rad-87™ Pulse CO-Oximeters with Masimo rainbow SET, and the Rad-5®, Rad-5v™ and Rad-8® Pulse Oximeters with Masimo SET.
* Hospitals must be located in a country where Masimo has a presence. Offer subject to rules and regulations of the country where the hospital is located. Full program details and eligibility requirements available at: www.masimo.com/heartprogram.
1 Secretary of Health & Human Services letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011.http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf
2 Alex R. Kemper, William T. Mahle, Gerard R. Martin, W. Carl Cooley, Praveen Kumar, W. Robert Morrow, Kellie Kelm, Gail D. Pearson, Jill Glidewell, Scott D. Grosse, R. Rodney Howell. "Strategies for Implementing Screening for Critical Congenital Heart Disease." Pediatrics; Volume 128, No. 5; November 2011; e1-w10 DOI: 10.1542/peds.2011-1317. http://pediatrics.aappublications.org/content/early/2011/10/06/peds.2011-1317.full.pdf
3 De-Wahl Granelli et al., "Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns." British Medical Journal (BMJ) January 2009; 338:a3037.
4 Ewer et al., "Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study." The Lancet 2011: Vol. 378; No. 9793; pp. 785-794.
Additional references: De-Wahl Granelli A,Mellander M, Sunnegardh J, Sandberg K, Östman-Smith I. "Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity." Acta Paediatr 2005;94:1590-6.http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2005.tb01834.x/abstract;jsessionid=E0F922B8E4E2950354BB77D36463ED96.d01t03?systemMessage=Wiley+Online+Library+will+be+disrupted+3+Sep+from+10-12+BST+for+monthly+maintenance.
Wren C, Richmond S, Donaldson L. "Presentation of congenital heart disease in infancy: implications for routine examination." Arch Dis Child Fetal Neonatal Ed. 1999;80:F49–F53. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1720871/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


-
-
This year marked Chapman University's inaugural TEDx (Technology, Entertainment & Design) event, which drew an impressive lineup of thought provoking talks from speakers who embody the theme of "icons, geniuses and mavericks," including our very own, Joe Kiani.
The Founder and CEO of Masimo was a featured speaker at the TEDxChapmanU event, where he inspirationally walked attendees through his life experiences and how they led to his belief that microscopic changes can bring forth a true revolution against injustice. This idea has guided his entrepreneurial spirit and gave birth to what he calls "micro-fixing."
The Micro-Fixing Revolution:
Deeply affected by the injustices he saw growing up, young Joe Kiani learned the value of igniting and becoming the change you want to see in the world. His concept of micro-fixing holds that small, microscopic changes can lead to micro-revolutions that give way to impactful, long-term revolutions that combat injustices. From smiling and saying 'hello' to being helpful to others, young Joe saw that even small fixes could create a groundswell of change and that every small change leads to the next—helping to dramatically change the landscape of the world.Learn more about Joe Kiani.
-
Watch Masimo Founder & CEO Joe Kiani at TEDx ChapmanU
Learn more about Masimo's Innovative Micro-Fixes in Healthcare…
-


Masimo Pronto-7® Wins GOLD "Stevie®" Award for Best New Health Product
 The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin, SpO2, pulse rate, and PI spot-check results.
The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin, SpO2, pulse rate, and PI spot-check results.
Irvine, California – September 20, 2012 – Masimo Corporation (NASDAQ: MASI) announced today that its Pronto-7® handheld noninvasive hemoglobin, arterial oxygen saturation (SpO2), pulse rate, and perfusion index spot-check testing device earned a 2012 GOLD Stevie® American Business Award for the Best New Product or Service in the Health & Pharmaceuticals category.
This marks the fourth industry award the innovative Pronto-7 has won since its commercialization last year. The 2012 Stevie Award winners were honored recently at a presentation ceremony at the Julia Morgan Ballroom, Merchants Exchange in San Francisco.
The Pronto-7 is a palm-sized device designed for quick-and-easy noninvasive total hemoglobin (SpHb®) spot-check testing, along with SpO2, pulse rate, and perfusion index. Extremely lightweight at approximately 296g, the Pronto-7 delivers total hemoglobin measurement results—a physiological parameter that previously required an invasive blood draw and time-consuming lab analysis—in under one minute. Offering needle-less and pain-free hemoglobin testing, the Pronto-7 is a cost-effective solution for immediate, on-the-spot hemoglobin testing that is raising the expectations of patients and clinicians alike to more advanced healthcare at the earliest moment of care.
"We are honored the Pronto-7 has earned a GOLD Stevie award, emerging atop an extremely competitive field this year," said Joe Kiani, Founder and CEO of Masimo. "The Pronto-7 incorporates some of Masimo's most advanced technology to help overcome many of the challenges and risks associated with invasive hemoglobin sampling for both clinicians and patients – enabling fast, accurate, painless, needle-free total hemoglobin measurements."
The Stevie Awards were created to honor and generate public recognition of the achievements and positive contributions of organizations and business people worldwide. Beginning with The American Business Awards in 2002, The International Business Awards in 2003, The Stevie Awards for Women in Business in 2004, and the Stevie Awards for Sales & Customer Service in 2006, the program's mission is to raise the profile of exemplary organizations and individuals among the press, the business community, and the general public. The Stevie has become one of the world's most coveted awards.
The American Business Awards are judged each year by more than 200 executives nationwide. Sponsors include several of the top business publishers and marketers.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Inova Health System Re-Ups System-wide Pulse Oximetry Contract with Masimo
Fairfax, Virginia & Irvine, California – September 19, 2012 – Inova Health System, one of the largest healthcare systems in Northern Virginia and the Washington D.C. metro area, today renewed its long-standing contract and commitment to Masimo SET® as its pulse oximetry standard-of-care.
The new five-year contract now includes Inova Loudoun Hospital – making Masimo pulse oximetry technologies and devices the standard-of-care for all care areas of five hospitals within the Inova Health System, including Inova Fairfax Hospital, rated No. 1 in the Washington, D.C. area on the 2012-2013 U.S. News & World Report honor roll. The contract also adds Masimo rainbow® SET® measurement capabilities with Masimo's handheld Rad-57™ Pulse CO-Oximeter™, designed to deliver rapid noninvasive blood measurements for improved patient assessments.
Inova Health System standardized on Masimo SET® pulse oximetry technology for Measure-Through Motion and Low Perfusion SpO2, pulse rate, and perfusion index measurements more than seven years ago because it virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events2. More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions – establishing the technology as an industry-leading pulse oximetry that substantially contributes to improved patient outcomes.
Today, the health system is expanding its relationship with Masimo and deploying two new Masimo rainbow® SET® Pulse CO-Oximetry measurements: carboxyhemoglobin (SpCO®) and methemoglobin (SpMet®), which help clinicians measure and detect carbon monoxide and methemoglobin blood levels without a painful needle stick or time-consuming blood lab analysis3,4. Masimo rainbow® SET® technology has been shown to have the highest pulse oximetry specificity (lowest rate of false alarms) and sensitivity (highest true alarm detection),2 as well as noninvasive patient monitoring solutions that allow clinicians to measure multiple blood constituents – including: oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), acoustic respiration rate (RRa™), and perfusion index (PI) in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
Thanks to Masimo's upgradable technology platform, Inova's existing Radical-7® bedside monitors can be upgraded to include additional Masimo rainbow® SET® measurements via a simple software upgrade that may be purchased at any time. No additional hardware or devices are required to obtain Masimo's full suite of noninvasive rainbow® SET® measurements – saving the health system time and money.
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Studyong>here.
3 Suner, S. Noninvasive Pulse CO-Oximetry Screening in the Emergency Department Identifies Occult Carbon Monoxide Toxicity. J Emerg Med 2007; 34(4):441-450.
4 Ash-Bernal R, Wise R, Wright SM. Acquired Methemoglobinemia. A Retrospective Series of 138 Cases at 2 Teaching Hospitals. Medicine. 2004; 83: 265-272.
About Inova Health System
Inova is a not-for-profit health care system located in the Washington, D.C. metropolitan area, serving over two million people with over 1,700 licensed beds based in Northern Virginia. Inova consists of five hospitals including the area's only Level 1 Trauma Center and Level 3 Neonatal Intensive Care unit. Inova encompasses many health services including the nationally and internationally recognized Inova Heart and Vascular Institute (IHVI), Inova Translational Medicine Institute (ITMI) on genomics, Inova Neuroscience Institute and Inova Children's Hospital. Inova's mission is to improve the health of the diverse community it serves through excellence in patient care, education and research. More information and statistics about Inova are at www.inova.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Easton Hospital Adds Masimo Patient SafetyNet™ System and rainbow®
Pulse CO-Oximetry for Noninvasive Patient Monitoring
Easton, Pennsylvania & Irvine, California – September 12, 2012 – Easton HospitalandMasimo (NASDAQ: MASI) today jointly announce that Easton Hospital is adding a new patient monitoring system – Masimo Patient SafetyNet™ and Masimo rainbow® Pulse CO-Oximetry technology for advanced noninvasive patient monitoring capabilities.
This conversion makes Easton Hospital the only full-service medical facility in the area equipped with Patient SafetyNet – a system that helps keep patients safer by noninvasively and continuously measuring and tracking the underlying physiological conditions of up to 80 patients on four floors with Signal Extraction Pulse Oximetry. The Patient SafetyNet system, clinically shown to decrease rapid response activations by 65% and costly ICU transfers by 48%,1 detects real-time changes and abnormalities that signal declining health status. When a patient's condition deteriorates, the system automatically sends wireless alerts directly to assigned clinicians – prompting a potentially lifesaving response at the patient's bedside.
In addition, clinicians at Easton Hospital will also benefit from the handheld Masimo Pronto® CO-Oximeter® for noninvasive spot-checking of total hemoglobin (SpHb®) along with SpO2, pulse rate, and perfusion index measurements – without the painful needle-stick, time-consuming blood draws, and associated lab waste. Pronto is powered by Masimo rainbow® SET® technology, shown to have the highest pulse oximetry specificity (lowest rate of false alarms) and sensitivity (highest true alarm detection),2 as well as noninvasive patient monitoring solutions that allow clinicians to measure multiple blood constituents – including: total hemoglobin (SpHb®).
The pulse oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow® SET® provides real-time and continuous blood constituent measurements and respiration rate that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
1 Taenzer AH, Pyke JB, McGrath SP, Blike GT. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study" Anesthesiology; February 2010; Vol 112, Issue 2, pp 282-287.
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91
About Easton Hospital
Easton Hospital is a 254-bed acute care teaching hospital serving more than 300,000 residents in Northampton County and the five surrounding counties in Pennsylvania and New Jersey. Founded in 1890, the Hospital offers an active Emergency Department which sees more than 35,000 annual visits; an cardiac care program including an accredited Chest Pain Center by the Society for Chest Pain Centers; a newly renovated Center for Orthopedics, Joint and Spine; a Bariatric Weight Loss program that has been designated a Center of Excellence in Bariatric Surgery; and the hospital's Easton Regional Cancer Center which is a Fox Chase Cancer Center Partner. In addition, the hospital maintains free-standing, fully-accredited residency training programs. For more information and a complete list of services, visit the hospital's website: www.easton-hospital.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Kimberly Golden-Benner
Easton Hospital
Phone: (610) 250-4953
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Host Investor Day on September 20 Live webcast begins at 8:00 a.m. ET
IRVINE, Calif., September 10, 2012 -- Masimo (NASDAQ: MASI) announced today that it will host an Investor Day meeting for institutional investors and analysts on Thursday, September 20, 2012, at 8:00 a.m. Eastern Time at The Pierre Hotel in New York. The program will include presentations by Masimo Chairman and Chief Executive Officer Joe Kiani and other members of the company's executive management team, as well as a clinical panel discussion and question-and-answer session.
A live audio webcast of the meeting will be available online from the investor relations page of the company's corporate website at www.masimo.com. To listen to the meeting via telephone, dial (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The conference code for both dial-in numbers is 26029818. An archive of the webcast will be available on Masimo's website through October 20, 2012. In addition, a telephonic replay of the meeting will be available through October 4, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use conference code 26029818.
A confirmed reservation is required to attend the meeting in person. To request an invitation, please visit the investor relations page at www.masimo.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

Phoenix Children's Hospital Standardizes on Masimo
Third-Largest Children's Hospital in the Nation Converts to Masimo's Breakthrough Measure-through Motion and Low Perfusion Pulse Oximetry
Phoenix, Arizona & Irvine, California – September 4, 2012 – Phoenix Children's Hospital, ranked among the top 50 children's hospitals across four medical specialties by U.S. News & World Report (2012-2013), and Masimo (NASDAQ: MASI) today announced the hospital's conversion to Masimo SET® pulse oximetry, offering clinicians advanced noninvasive and continuous patient monitoring designed to improve patient outcomes and reduce cost of care.
"As one of the leading children's hospitals in the nation, we take to heart our obligation to provide the best care for all of our young patients," said Christine Tenaglia, Respiratory Care Director at Phoenix Children's Hospital. "Our conversion to Masimo's state-of-the-art pulse oximetry arms our clinicians with monitoring equipment that can measure accurately even when patients move or have low perfusion – capabilities unmatched by competing pulse oximeters. We tried other pulse oximeters, but have found Masimo's technology to be more accurate, even under demanding conditions."
Phoenix Children's five-year contract for Masimo SET® pulse oximetry includes the bedside Rad-87® pulse oximeter and the handheld or bedside Radical-7™ Pulse CO-Oximeter®. The hospital also plans to upgrade to Masimo rainbow® technology to noninvasively monitor total hemoglobin (SpHb®).
Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events2. More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions – establishing the technology as the industry-leading pulse oximetry that substantially contributes to improved patient outcomes.
Phoenix Children's – with more than 12,500 annual admissions, 57,000+ emergency room visits, and more than 15,200 specialty and urgent care visits – offers world-class care in more than 40 subspecialty fields of pediatric medicine, including six Centers of Excellence.
"We treat more young patients than any hospital in our area," said Tenaglia. "Masimo pulse ox technology allows us to obtain accurate readings, even on our most fidgety patients – an important factor when dealing with the sheer volume we see on any given day. Masimo's technology also has decreased the frequency of false alarms. That means we can do our jobs better and more efficiently, rather than responding to nuisance alarms."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Studyhere
About Phoenix Children's Hospital
Phoenix Children's Hospital, ranked in U.S. News & World Report's Best Children's Hospitals, is Arizona's only licensed children's hospital, providing world-class care in more than 40 pediatric specialties to children from throughout the state and region. Phoenix Children's is in the midst of a major expansion to meet the needs of the Southwest's rapid population growth. The signature element of the expansion is a new 11-story, 750,000-square-foot tower which will enable the hospital to grow from 345 licensed beds today to a total of 626 licensed beds once the project is complete. The hospital's expansion also includes an aggressive physician recruitment effort and new satellite centers in high growth areas of the Valley. For more information, visit the hospital's web site at www.phoenixchildrens.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jane Walton
Phoenix Children's Hospital
Phone: (602) 933-5871
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Princeton's University Medical Center Deploys Masimo Patient SafetyNet™
New State-of-the-Art Hospital Equipped with Masimo SET® Pulse Oximetry for Oxygenation Monitoring and rainbow® Acoustic Monitoring for Ventilation Monitoring
Irvine, California & Plainsboro, New Jersey – August 28, 2012 – Masimo (NASDAQ: MASI) and the University Medical Center of Princeton at Plainsboro, N.J. (UMCPP), announced today the successful installation of Masimo Patient SafetyNet™, clinically shown to reduce rapid response activations, intensive care unit (ICU) transfers, and deaths related to opioid-induced respiratory depression, while offering significant cost savings opportunity.
UMCPP joins a growing list of distinguished hospitals using Masimo Patient SafetyNet, which tracks the underlying physiological conditions of patients, and detects changes and abnormalities that signal declining health status in real time. When a patient's condition deteriorates, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside. The Masimo Patient SafetyNet system consists of Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, choice of patient-tolerant and easy-to-use ventilation monitoring with rainbow® Acoustic Monitoring or standard capnography, and remote monitoring and notification to keep clinicians connected to patients.
This year the clinical care provided by UMCPP received an "A" in patient safety from The Leapfrog Group, an independent, national nonprofit working to improve the safety, quality, and affordability of healthcare. More than 2,600 hospitals were evaluated – only about one-fourth of those earned an "A."
"We are committed to patient safety and incorporating new technologies and practices that will ensure that we continue to be considered among the safest hospitals in the nation," said Linda F. Sieglen, MD, MMM, a board certified anesthesiologist and Senior Vice President of Medical Affairs for Princeton HealthCare System, which includes UMCPP.
Improved care does not need to come at a higher cost. Clinicians at Dartmouth Hitchcock Medical Center, for instance, reported that using Patient SafetyNet on just one floor reduced ICU transfers and created an annual opportunity-cost savings of $1.48 million.1
Michael O'Reilly, MD, Chief Medical Officer of Masimo, stated: "We are excited to join UMCPP in its mission to provide safe, cost-efficient care, and to help offer its patients and their families peace of mind. With Masimo technologies on the general floor, clinicians can be confident their patients are being watched even when they aren't at the bedside, while families can be assured their loved ones are safe."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
About University Medical Center of Princeton at Plainsboro
A unit of Princeton HealthCare System, University Medical Center of Princeton at Plainsboro opened in May 2012 as the hub of a 171-acre health campus in Plainsboro, N.J. UMCPP is a University Hospital Affiliate of the University of Medicine & Dentistry of New Jersey—Robert Wood Johnson Medical School, a Clinical Research Affiliate of The Cancer Institute of New Jersey, an accredited oncology-teaching program of the American College of Surgeons' Commission on Cancer (CoC) and recipient of the CoC's Outstanding Achievement Award. Through a partnership with The Children's Hospital of Philadelphia (CHOP), UMCPP offers enhanced pediatric services, with CHOP physicians working onsite to provide pediatric emergency consultation, inpatient pediatric care and neonatal care. Visit www.princetonhcs.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Andy Williams
University Medical Center of Princeton at Plainsboro
(609) 252-8785
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Joint Commission Issues Sentinel Event Alert on Opioid-Induced Respiratory Depression Recommends Continuous Monitoring of Oxygenation and Ventilation of Post-Surgical Patients
Irvine, California – August 13, 2012 – Masimo (NASDAQ: MASI) announced today that The Joint Commission has issued a Sentinel Event Alert on safe use of opioids in hospitals with recommendations to implement better dosing along with oxygenation and ventilation monitoring in post-surgical patients. The Masimo Patient SafetyNet™ System, consisting of Measure-through Motion and Low Perfusion oxygenation monitoring (Masimo SET® Pulse Oximetry or rainbow SET® Pulse CO-Oximetry), choice of easy-to-use ventilation monitoring with rainbow® Acoustic Monitoring or standard capnography, and remote monitoring and clinician notification to keep clinicians connected to patients, can help hospitals comply with The Joint Commission recommendations. The Patient SafetyNet System is also the only technology clinically shown to reduce rapid response activations, intensive care unit (ICU) transfers, and deaths related to opioid-induced respiratory depression.
While opioid use is safe for most patients, opioid analgesics are associated with adverse effects1,2,3 and cause respiratory depression in 0.5% of post-surgical patients, who often receive them for pain management.4,5,6 Opioid analgesics rank among the drugs most frequently associated with adverse drug events, the Sentinel Event Alert noted. Of opioid-related adverse drug events – including deaths – that occurred in hospitals and were reported to The Joint Commission's Sentinel Event database (2004-2011), 47% were wrong dosing medication errors, 29% were related to improper monitoring of the patient, and 11% were related to other factors including excessive dosing, medication interactions, and adverse drug reactions.
Similar to the 2011 Anesthesia Patient Safety Foundation's recommendations,7 the Sentinel Event Alert recommended continuous monitoring (instead of spot checks) of both oxygenation and ventilation. Oxygenation monitoring is possible with Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, which is ideal for the general floor where patients are often moving and the nursing staff does not have the time to respond to false alarms. Ventilation monitoring is possible with Masimo's rainbow® Acoustic Monitoring, which provides continuous respiration rate through an acoustic sensor on the neck, or standard capnography, which provides continuous respiration rate and end-tidal carbon dioxide (EtCO2) through a nasal cannula. While capnography is considered the clinical standard for unconscious, mechanically ventilated patients undergoing surgery, in conscious patients the nasal cannula is often not well tolerated. In addition, the nasal cannula can become dislodged or unable to pick reliable signals when patients breathe through their mouths, requiring additional nursing staff time to manage.
In a 2010 landmark study published in Anesthesiology, clinicians at Dartmouth Hitchcock Medical Center reported that use of Masimo SET® pulse oximetry and Patient SafetyNet in a single orthopedic post-surgical unit led to a 65% decrease in rapid response activations and a 48% decrease in ICU transfers.8 A follow-up analysis appearing in the Spring-Summer 2012 issue of the Anesthesia Patient Safety Foundation newsletter validated the original findings by showing similar clinical results on two additional post-surgical floors.9 Importantly, Dartmouth Hitchcock also reported that no patients on the original orthopedic unit have died or suffered irreversible brain injury as a result of respiratory depression from opioids since the Patient SafetyNet system was instituted in December of 2007.
Cost of care is an increasingly important factor in today's healthcare environment. Dartmouth Hitchcock also reported that using Patient SafetyNet on just one floor reduced ICU transfers and created an annual opportunity-cost savings of $1.48 million. This demonstrates that Patient SafetyNet not only helps clinicians improve clinical outcomes, but that it does so in a cost-effective manner with benefits exceeding the cost of equipment, sensors, and training time.
Joe Kiani, Founder & CEO of Masimo, stated: "Since our founding in 1989, our mission has been to improve patient outcomes and reduce cost of care by taking noninvasive monitoring to new sites and applications. From the beginning, we observed that conscious patients were not getting the best care because they couldn't be monitored reliably due to false pulse oximetry measurements and alarms that occurred with the natural movement of their bodies. In areas like the general floor, countless patients have tragically died due to this prior limitation of pulse oximeters. By solving the motion artifact limitation with SET®, Masimo has ushered in an era where prestigious groups like the Anesthesia Patient Safety Foundation and The Joint Commission can now recommend monitoring of patients in new sites like the general floor, where previously patients were needlessly dying due to undetected physiologic deterioration."
1 Vila H Jr, Smith RA, Augustyniak MJ: The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: Is patient safety compromised by treatment based solely on numerical pain ratings? Anesthesia and Analgesia, 2005;101:474-80
2 Emergency department visits involving nonmedical use of selected prescription drugs – United States, 2004-2008. Morbidity and Mortality Weekly Report 2010, 59:705-709
3 Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Substance abuse treatment admissions involving abuse of pain relievers: 1998 and 2008, http://oas.samhsa.gov/2k10/230/230PainRelvr2k10.cfm (accessed October 28, 2011)
4 McPherson ML: Strategies for the management of opioid-induced adverse effects. Advanced Studies in Pharmacy, 2008;5(2):52-57
5 Jarzyna D, et al: American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing, 2011;12(3):118-145.e10
6 Pasero C, M McCaffery: Pain assessment and pharmacologic management. Chapter 12 – Key Concepts in Analgesic Therapy, and Chapter 19 – Management of opioid-induced adverse effects. St. Louis, Mosby Elseveir, 2011
7 Stoelting RK, Weinger MB. Dangers of Postoperative Opioids -- Is There a Cure? APSF Newsletter, 2009;24:25-26. http://www.apsf.org/newsletters/html/2009/summer/index.htm
8 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.
9 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms all other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow SET® technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo's Noninvasive Pleth Variability Index as Accurate as Invasive Stroke Volume Variation for Fluid Responsiveness Assessment in Cardiac Surgery
Irvine, California – August 7, 2012 – Masimo (NASDAQ: MASI) announced today a new study in the Journal of Anesthesia demonstrates that for fluid responsiveness assessment, Masimo's noninvasive Pleth Variability Index (PVI®) is as accurate as invasive stroke volume variation (SVV) in patients undergoing cardiopulmonary bypass surgery.1 In the study conducted at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany, researchers evaluated 18 patients who underwent elective cardiopulmonary bypass surgery. All patients had a central venous catheter as well as a femoral arterial catheter monitored with the PiCCO monitor (PiCCO2; Pulsion Medical Systems) for continuous measurement of SVV and intermittent assessment of cardiac index. PVI was measured using the Masimo Radical-7® monitor.
Fluid responsiveness was defined as a >10% increase in cardiac index after fluid administration and non-responsiveness was defined as <10% increase in cardiac index after fluid administration. Out of 22 fluid challenges, four resulted in a significant increase in cardiac index, while 18 did not. The optimal threshold value for fluid responsiveness was ≥11% for SVV and ≥16% for PVI. Both PVI and SVV correctly identified each of the four responders (100% sensitivity), while PVI identified 16 of 18 non-responders (89% sensitivity) and SVV identified 13 of 18 non-responders (72% sensitivity). Receiver operator characteristic curve analysis, which takes into account both sensitivity and specificity, showed a 0.95 area under the curve (AUC) for PVI and 0.87 AUC for SVV (p=0.31 for difference between PVI and SVV). Central venous pressure was a poor predictor of fluid responsiveness, with an AUC of 0.19.
The authors stated that both PVI and SVV are "much more valuable for prediction of volume responsiveness than CVP" and that PVI "is of high value, especially regarding sensitivity and negative predictive value. Thus, volume responsiveness is not to be expected when PVI is low."
Fluid administration is usually the first intervention in hemodynamically unstable patients to improve status and enable end organ preservation.2 Fluid is given to increase a patient's cardiac output, but only about half of critically ill patients actually increase cardiac output after fluid administration.3 Unnecessary fluid administration should be avoided because it's associated with increased morbidity and mortality.4 Clinical decision making is challenging using traditional invasive measurements such as central venous pressure, stroke volume/index, cardiac output/index, and pulmonary capillary wedge pressure, as they have not been shown to predict fluid responsiveness.5 Newer, dynamic indices such as SVV and pulse pressure variation can accurately predict fluid responsiveness and improve outcomes,6 but are invasive and/or complex and have a high procedural cost, estimated to be about $225 with an SVV monitoring system in one cost analysis.7 PVI is also a dynamic measurement of changes over respiratory variations, but does so with the Masimo SET plethysmograph waveform instead of the arterial waveform. In contrast to other available methods, PVI is noninvasive, easy to use, and has no incremental procedural cost because pulse oximetry monitoring is already performed on all surgical and intensive care patients. No other pulse oximetry technology provides a parameter like PVI. Once Masimo SET® pulse oximetry or rainbow® Pulse CO-Oximetry™ with PVI is available in a hospital, it can be utilized in all patients.
Previously, PVI has been shown to help clinicians assess fluid responsiveness in adult surgical and intensive care patients under mechanical ventilation.8,9,10 In addition to the present study comparing PVI to SVV from the PiCCO invasive catheter from Pulsion, two previous studies have compared PVI to SVV from the FloTrac invasive catheter from Edwards Lifesciences and showed similar results.11,12 Masimo PVI has also been shown to help clinicians improve surgical fluid management to decrease patient risk in a randomized controlled trial.13
The United Kingdom's National Health Service advises hospitals to use Intraoperative Fluid Management Technologies to improve patient outcomes and decrease costs. In June 2012, the NHS included PVI in its new Intra Operative Fluid Management pack, which serves as a guide for hospitals wishing to implement fluid responsiveness monitoring.14
Michael O'Reilly, MD, Chief Medical Officer of Masimo, stated: "Masimo is indeed improving patient outcome and reducing cost of care with its breakthrough measurements, such as PVI. Masimo is the only pulse oximetry technology to offer automated assessment of fluid responsiveness with PVI, along with other breakthrough measurements available with rainbow® technology such as noninvasive and continuous hemoglobin (SpHb®) to help clinicians reduce unnecessary blood transfusions."
1 Haas S., Trepte C., Hinteregger M., Fahje R., Sill B., Herich L., Reuter D.; "Prediction of volume responsiveness using pleth variability index in patients undergoing cardiac surgery after cardiopulmonary bypass." J Anesth. 2012 May; DOI 10.1007/s00540-012-1410-x.
2 Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis
and septic shock: 2008. Crit Care Med 2008;
3 Brandstrup B, Tonnesen H, Beier-Holgersen R, et al: Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann Surg 2003; 238:641–648
36:296–327
4 Michard F, Teboul JL: Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest 2002; 121:2000–2008
5 Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid
responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit Care Med 2009; 37:2642–2647.
6 Bundgaard-Nielsen M et al. Acta Anaesthesiol Scand. 2007; 51(3):331-40
7 Manecke G. Edwards FloTrac™ sensor and Vigileo™ monitor: easy, accurate, reliable cardiac output assessment using the arterial pulse wave. Expert Rev Med Devices. 2005;2(5):523-527.
8 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia August 2008; 101(2):200-6. http://www.ncbi.nlm.nih.gov/pubmed/18522935.
9 Loupec T., Nanadoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically Ill Patients." Crit Care Med. 2011 Feb;39(2):294-9.
10 Hood J.A., Jonathan R., Wilson T. Anesth. Analg. Pleth Variability Index to Predict Fluid Responsiveness in Colorectal Surgery. 2011;113:1058–63.
11 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology June 2010; 27(6):555-61. http://www.ncbi.nlm.nih.gov/pubmed/20035228.
12 Fu Q., Mi WD., Zhang H. "Stroke Volume Variation and Pleth Variability Index to Predict Fluid Responsiveness During Resection of Primary Retroperitoneal Tumors in Hans Chinese." BioScience Trends. 2012; 6(1):38-43 DOI: 10.5582/bst.2012.v6.1.38. http://www.ncbi.nlm.nih.gov/pubmed/22426102.
13 Forget P, Lois F, De Kock M. "Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesthesia & Analgesia. 2010 Oct;111(4):910-4. http://www.anesthesia-analgesia.org/content/early/2010/08/12/ANE.0b013e3181eb624f.abstract?ct=ct.
14 http://www.ntac.nhs.uk/NewsAndEvents/IOFM_Technology_Adoption_Pack_Published.aspx
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms all other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow SET® technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Reports Second Quarter 2012 Financial Results
Q2 2012 Highlights (compared to Q2 2011):
- Product revenue rose 12% to $115.3 million
- Masimo rainbow revenue rose 7% to $9.7 million, including 48% increase in SpHb revenue
- Shipped 37,300 Masimo SET® and Masimo rainbow® SET units
- Net income rose to $17.7 million, with EPS of $0.30
Irvine, California, August 1, 2012 – Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended June 30, 2012.
Masimo's total second quarter revenue, including royalties, rose 12% to $122.8 million, compared to $109.6 million for the second quarter of 2011. Second quarter 2012 product revenue rose 12% to $115.3 million, compared to $102.6 million for the second quarter of 2011. The company's worldwide end-user business grew 16% in the second quarter of 2012 and represented 85% of product revenue. OEM sales, which accounted for 15% of total product revenue, declined 5% compared to the same period in 2011. Revenue from sales of Masimo rainbow products rose 7% to $9.7 million in the second quarter, compared to $9.1 million for the second quarter of 2011. Second quarter 2012 rainbow revenue included a 48% increase in total hemoglobin (SpHb) sales, compared to the second quarter of 2011.
Net income for the second quarter was $17.7 million, or $0.30 per diluted share, compared to net income of $17.0 million, or $0.28 per diluted share, in the second quarter of 2011. Net income for the second quarter of 2012 included an income tax benefit resulting from the conclusion of a prior year tax audit, which increased the company's diluted earnings per share by $0.03.
During the second quarter, the company shipped approximately 37,300 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, unchanged from the same prior-year period. Masimo estimates its worldwide installed base as of June 30, 2012 to be 1,033,000 units, up 12% from 922,000 units as of July 2, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "With 12% growth in our product revenue and worldwide installed base, Masimo continued to significantly outpace market growth. Our end-user, or direct, business delivered even stronger growth at 16% versus the second quarter of 2011, fueled by increased consumable sales in the U.S., Europe, Asia and other regions. This performance is a testament to the superiority of our gold standard Masimo SET pulse oximetry technology and the growing appeal of our breakthrough Masimo rainbow SET platform, which is the first and only platform to provide continuous, noninvasive monitoring of carbon monoxide, total hemoglobin, methemoglobin, fluid responsiveness and respiration rate."
As of June 30, 2012, cash and cash equivalents were $121.5 million, compared to $129.9 million as of December 31, 2011. The change reflects primarily net cash generated from operations, offset by $7.2 million in cash used to purchase the assets of Spire Semiconductor in March 2012 and $26.3 million in cash used to repurchase shares of Masimo common stock. In the second quarter of 2012, the company completed its full three million share repurchase program authorized by the Board of Directors in August 2011.
Financial Guidance
Masimo today announced the acquisition of PHASEIN, AB and, as a result, is providing updated 2012 financial guidance. Masimo now expects total revenue of approximately $494 million, including $466 million in product revenue and $28 million in royalty revenue. This compares to prior guidance of $458 million in product revenue and $28 million in royalty revenue. As a result of the acquisition, Masimo estimates a $0.04 dilutive impact on its previous 2012 EPS guidance of approximately $1.15. In addition, Masimo is also updating its guidance for the remainder of 2012 to reflect slightly lower product gross profit margin expectations, a slightly higher effective tax rate and the inclusion of first half 2012 foreign exchange losses. The impact of these revisions to guidance is offset by the $0.03 tax benefit the company recognized in the second quarter of 2012. As a result of the acquisition and the other assumptions noted above, the company now expects 2012 GAAP earnings per share to be approximately $1.11, compared to prior guidance of approximately $1.15.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 10077057. After the live webcast, the call will be available on Masimo's website through September 1, 2012. In addition, a telephonic replay of the call will be available through August 15, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 10077057.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Media Contact:
Mike Drummond
949-297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
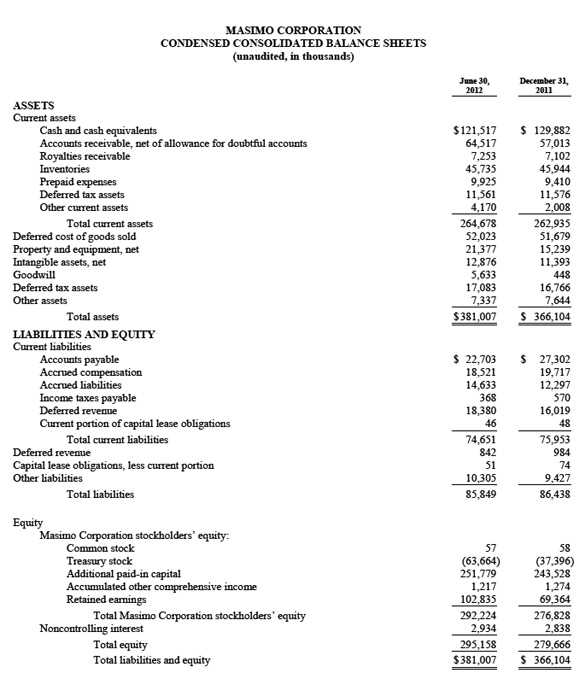
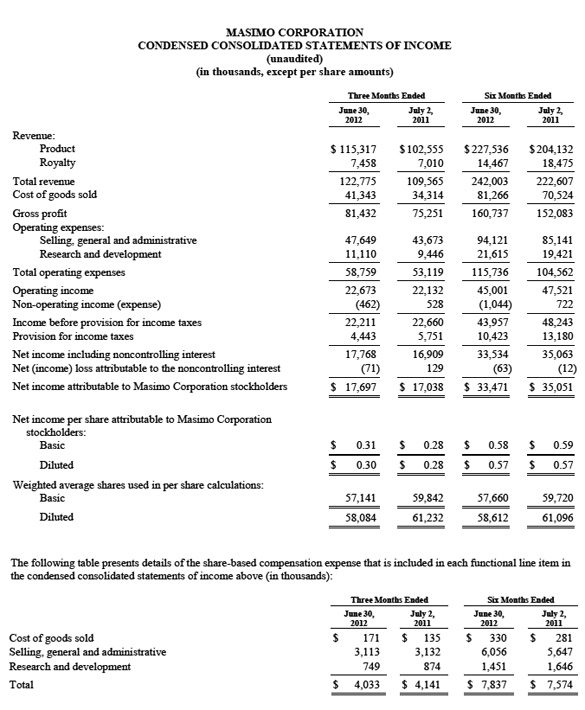



Masimo Enters Noninvasive Multigas Monitoring, including Capnography, with Acquisition of PHASEIN
Irvine, California – August 1, 2012 – Masimo (NASDAQ: MASI), the inventor of rainbow® Pulse CO-Oximetry™, and SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced it has acquired PHASEIN AB (Stockholm, Sweden), a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions.
The acquisition of PHASEIN's multigas technologies complements Masimo's breakthrough innovations for patient monitoring with a portfolio of products ranging from OEM solutions for external "plug-in-and-measure" gas analyzers and integrated modules to handheld devices. With multiple measurements delivered through either mainstream or sidestream options, Masimo customers can now benefit from CO2, N2O, O2, and anesthetic agent monitoring in many hospital environments, such as operating rooms, procedural sedations and intensive care units.
Joe Kiani, Masimo CEO and Chairman of the Board, stated, "We are confident that the best technology always wins if clinicians are given the choice. PHASEIN has developed leading-edge infrared multispectral technologies for measuring respiratory gases. In our assessment, PHASEIN has the most accurate gas measurements, the widest array of measurements, and the technology most suited for easy and flexible integration in both Masimo and OEM products. PHASEIN's impressive list of OEM customers and innovative technology portfolio for monitoring CO2, N2O, O2, and anesthetic agents is a perfect addition to Masimo's mission to take noninvasive patient monitoring to new sites and applications. We see multigas measurements as a half-billion dollar global market opportunity and are proud to welcome the PHASEIN team to the Masimo team."
PHASEIN CEO Robert Zyzanski will remain the CEO of the company. "Masimo's pursuit of innovation for the betterment of patient care is bar none. The opportunity to become part of Masimo is yet another validation of our advanced capnography and gas monitoring technologies," Zyzanski said. "We look forward to continuing to develop industry-leading solutions with our new colleagues, as we strive to leverage the synergies of our complementary technologies to improve patient outcomes and reduce the cost of care worldwide."
Masimo paid approximately $30.4 million in cash to purchase PHASEIN. No other terms were disclosed. The estimated impact of the acquisition on Masimo's 2012 financial results will be included in the company's second quarter earnings announcement, set for release after the market close today.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995, including the opportunities Masimo expects from the acquisition; the planned integration of PHASEIN's technologies with Masimo's technologies; the prospective benefits of the acquisition to Masimo's customers; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and demand for our technologies . These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to: the successful integration of PHASEIN and Masimo personnel and technologies; our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Study Reveals Wide Variation in Blood Transfusion Practices During Surgery Opportunity for Masimo Noninvasive and Continuous Hemoglobin Monitoring to Help Optimize Transfusion Decisions
IRVINE, California – July 26, 2012 – According to a new study in the July 2012 print edition of Anesthesiology, blood transfusion, the most common procedure performed in U.S. hospitals1, has wide variation in frequency by surgical procedure and physician as well as wide variation in the hemoglobin trigger used to help decide whether to transfuse.2 The study also showed a significant number of transfusion decisions are made without laboratory hemoglobin measurements. The research adds to the growing clinical evidence highlighting the need for improved blood-management strategies. It also underscores the opportunity for noninvasive and continuous total hemoglobin (SpHb®) monitoring from Masimo (NASDAQ: MASI) to facilitate optimal transfusion decision making to improve patient safety and reduce costs.
In the study, conducted at Johns Hopkins Hospital in Baltimore, Maryland, researchers collected data on 48,086 surgical patients over 18 months and evaluated blood transfusion frequency and hemoglobin triggers by surgical procedure and physician. A total of 2,981 patients (6.2%) received an intra-operative red blood cell transfusion, with two-thirds of those patients receiving two or more units. Transfusion rates varied up to threefold between different physicians performing the same procedure (p<0.05). The average transfusion hemoglobin trigger used to determine need for blood transfusion varied widely with both surgeons (7.2 g/dL to 9.8 g/dL, p=0.001 and anesthesiologists (7.2 g/dL to 9.6 g/dL, p=0.001). The ending hemoglobin values after the last recorded transfusion also varied widely for both surgeons (8.8 g/dL to 11.8 g/dL, p=0.001) and anesthesiologists (9.0 g/dL to 11.7 g/dL, p=0.0004). A recent laboratory hemoglobin measurement was not available when 31% of transfusion decisions were made.
Blood transfusions carry risks. In a previous meta-analysis of 45 studies evaluating the risks of blood transfusion, 42 studies showed a significant link to mortality, infection, or adult respiratory distress syndrome.3 In contrast to the historical belief that withholding transfusions harms patients, multiple randomized controlled trials have now proven that restrictive transfusion practice is safe.4,5,6 This has led recent transfusion guidelines to focus transfusion decisions on the overall patient condition and to suggest hemoglobin transfusion triggers of 6-7 g/dL for most patients and above 7 g/dL only in select, high-risk patients.7,8,9
Blood transfusions are also one of the largest cost centers in hospitals. While the material cost of blood ranges from $200 to $300 per unit, the additional costs from storage, labor, and waste result in an actual cost per unit between $522 and $1,183.10 In addition to the cost of blood itself, each unit of blood transfused increases the cost of care, with even higher costs incurred when patients are transfused at higher hemoglobin levels.11
A recent systematic evaluation of 494 studies concluded that 59% of transfusions were "inappropriate" based on their impact on patient outcomes.12 The risks and costs of blood transfusion paired with unnecessary transfusions led the Joint Commission in 2011 to introduce new patient blood management measures that hospitals are being encouraged to adopt as a quality indicator.13 The new measures include recording the clinical indication for transfusion along with the hemoglobin value of the patient prior to each unit transfused. With the need to stem rising health care expenditures, the Joint Commission and the American Medical Association have targeted blood transfusion procedures as one of the top procedures to reduce in a "National Summit on Overuse" scheduled for September 2012.14
There is no doubt that clinicians desire the best care for their patients without unnecessary costs, but they are also limited in their precise ability to determine need for transfusion with existing tools. Estimates of blood loss in the operating room can be inaccurate. Researchers at Duke University recently reported estimated surgical blood loss exceeded measured blood loss by more than 40% (860mL vs. 611 mL, p< 0.0001).15 The likely reason for this discrepancy is the inability to accurately estimate blood loss based on visual inspection of blood and fluid in suction canisters and surgical sponges. While estimating blood loss is challenging and laboratory hemoglobin results are only availably intermittently and are often delayed, transfusion decisions are made in real time. Acknowledging these challenges, the Duke Researchers stated: "Use of bedside hemoglobin concentration devices and continuous, noninvasive hemoglobin monitors may improve transfusion decisions."
Masimo's breakthrough SpHb measurement allows clinicians to noninvasively and continuously monitor hemoglobin. Results of an earlier randomized controlled trial conducted by researchers at Massachusetts General Hospital and Harvard Medical School showed that SpHb helped anesthesiologists reduce the frequency of blood transfusion by 87% (from 4.5% to 0.6%, p=0.03) and quantity of blood by 90% (from 0.1 to 0.01 units per patient, p<0.0001) in 327 patients undergoing orthopedic surgery.16
Dr. Aryeh Shander, Executive Medical Director at the Institute for Patient Blood Management & Bloodless Medicine Surgery and Chief of Anesthesiology and Critical Care Medicine at Englewood Hospital & Medical Center in New Jersey, stated: "The ability of Masimo's noninvasive hemoglobin technology to continuously monitor hemoglobin during surgeries can offer earlier, real-time information that can result in diagnosis leading to interventions other than transfusion. And fewer unnecessary transfusions can mean improved patient outcomes."
This year Masimo launched the Blood Transfusion Related Cost Reduction guarantee program (BTR-CR, "Better Care") to help hospitals improve patient care and reduce costs. BTR-CR guarantees that a hospital's blood transfusion-related cost reductions will be greater than the cost of SpHb monitoring. For more information, contact 888-44BTRCR or visit BTR-CR.
1 AHRQ, Center for Delivery, Organization, and Markets, Healthcare Cost and Utilization Project, Nationwide Inpatient Sample, 1997 and 2007.
2 Steven M. Frank, M.D., Will J. Savage, M.D., Jim A. Rothschild, M.D., Richard J. Rivers, M.D., Paul M. Ness, M.D., Sharon L. Paul, B.S., M.S., John A. Ulatowski, M.D., Ph.D., M.B.A. "Variability in Blood and Blood Component Utilization as Assessed by an Aesthesia Information Management System." Anesthesiology, July 2012 - Volume 117 - Issue 1 - p 99–106 doi: 10.1097/ALN.0b013e318255e550
3 Marik, P. E. and H. L. Corwin (2008). "Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature." Crit Care Med 36(9): 2667-74.
4 Carson, J. L., M. L. Terrin, et al. (2011). "Liberal or restrictive transfusion in high-risk patients after hip surgery." N Engl J Med 365(26): 2453-62.
5 Hebert, P. C., G. Wells, et al. (1999). "A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group." N Engl J Med 340(6): 409-17.
6 Hajjar, L. A., J.-L. Vincent, et al. (2010). "Transfusion Requirements After Cardiac Surgery: The TRACS Randomized Controlled Trial." JAMA 304(14): 1559-1567.
7 American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies: Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006; 105:198 –208
8 Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA, American College of Critical Care Medicine of the Society of Critical Care Medicine, Eastern Association for the Surgery of Trauma Practice Management Workgroup: Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. Crit Care Med 2009; 37:3124 –57
9 Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, International Consortium for Evidence Based Perfusion, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG: 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011; 91:944 – 82
10 Shander, A.,A. Hofmann, et al. "Activity-based costs of blood transfusions in surgical patients at four hospitals." Transfusion 50(4): 753-65.
11 Murphy, G. J., B. C. Reeves, et al. (2007). "Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery." Circulation 116(22): 2544-52.
12 Shander, A., A. Fink, et al. (2011). "Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes." Transfus Med Rev 25(3): 232-246 e53.
13 Gammon HM, Waters JH, Watt A, Loeb JM, Donini-Lenhoff A: Developing performance measures for patient blood management.
Transfusion 2011; 51:2500 –9.
14 Joint Commission Perspectives. The Joint Commission Continues to Study Overuse Issues. Volume 32, Number 5, 2012 : 4-8(5).
15 Hill, S., Broomer, B Stover, J, White, W. (2011). Accuracy of estimated blood loss in spine surgery. American Society of Anesthesiologists Annual Conference, San Diego, CA
16 Ehrenfeld JM, Henneman JP, Sandberg WS. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." American Society Anesthesiologists. 2010;LB05
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Masimo Signs Pulse Oximetry Distribution Deal with MWI Veterinary Supply
Agreement Marks Major National Push into the Animal Healthcare Market
Irvine, California – July 23, 2012 – Masimo (NASDAQ: MASI) announced today that MWI Veterinary Supply, Inc. (NASDAQ: MWIV), one of the largest national distributors of medical supplies and equipment to veterinarians, will for the first time distribute Masimo products.
Masimo's Radical-7® Pulse CO-Oximeter with upgradable rainbow® technology and Masimo's handheld Rad-5® and portable Rad-8® with SET Measure-through Motion and Low Perfusion pulse oximetry will be available to MWI's U.S. customer base of veterinarians and animal healthcare providers in all 50 states.
"Veterinarians understand Masimo's breakthrough pulse oximetry technology delivers accurate measurements, even during patient movement and low perfusion," said Kristin Krawczyk, MWI National Equipment Program Manager. "We look forward to working with Masimo to expand its reach into the veterinary market and to meet the upsurge in demand for its breakthrough pulse oximetry technology."
The American Pet Products Association estimates that 62% of all U.S. households – about 73 million homes – own a pet. Veterinary Pet Insurance Co. (VPI) reports that nationwide, its policyholders spent more than $46 million in 2011 treating the 10 most common medical conditions afflicting their pets. Masimo's technology is designed to provide clinicians oxygen saturation (SpO2) measurements under the most challenging conditions – patient motion and low perfusion, which are common in animal healthcare. Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events.2
"Masimo pulse oximeters have far fewer drop-offs and subsequent false alarms in post-op," said Bob Klostermann, a veterinarian and consultant at the University of Wisconsin, Madison. "Masimo's equipment works consistently and gives the entire staff and surgeon a lot more peace of mind with consistent, accurate readings and minimal drop-off alarms."
"As one of the largest and most respected distributors of animal health products in the nation, MWI offers Masimo an opportunity to better reach veterinarians," said Masimo Director of Strategic Marketing, Alternate Care, Gary Marston. "Animal healthcare represents an important and growing business for Masimo, and we look forward to working with MWI to help clinicians improve outcomes for their four-legged, feathered and reptilian patients."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
About MWI Veterinary Supply, Inc.
MWI is a leading distributor of animal health products across the United States of America and United Kingdom. MWI sells both companion animal and production animal products including pharmaceuticals, vaccines, parasiticides, diagnostics, micro feed ingredients, supplies, pet food, capital equipment and nutritional products. MWI also is a leading innovator and provider of value-added services and technologies used by veterinarians and producers. For more information about MWI, please visit www.mwivet.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Stacy Phillips
MWI Veterinary Supply Co.
(208) 955-9477
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report Second Quarter 2012 Financial Results after Market Close on August 1 Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, California, July 18, 2012 – Masimo (NASDAQ: MASI) announced today that it will release second quarter financial results for the period ended June 30, 2012, after the market closes on August 1, 2012. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 10077057. After the live webcast, the call will be available on Masimo's website through September 1, 2012. In addition, a telephonic replay of the call will be available through August 15, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code10077057.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Pulse Oximetry Screening Hailed as the 'New Milestone' to Combat Critical Congenital Heart Disease - Signal Extraction Technology Leading the Way in Large, Independent Clinical Studies
Irvine, California – July 17. 2012 – Pulse oximetry screening is a "new milestone" in the war on critical congenital heart disease (CCHD), one of the most common causes of infant deaths worldwide, according to a recent editorial in The Lancet and accompanying meta-analysis of studies including 229,421 babies.
The meta-analysis by Drs. Andrew Ewer and Shakila Thangaratinam, and colleagues Kiritrea Brown, Javier Zamora, and Khalid S Khan, pooled the results of 13 separate studies and reported the sensitivity of pulse oximetry to detect babies with CCHD at 76.5% (67.7 – 83.5%) and specificity to identify babies without CCHD at 99.9% (99.7 – 99.9%), a significant improvement over existing approaches. The authors noted that three recent large studies1,2,3 "used motion-tolerant sensors, which function in states of low perfusion", a reference to the use of Masimo Signal Extraction Technology (SET).
The Lancet editorial noted that each year in the United States, some 40,000 babies are born with congenital heart disease and about 4,800 have CCHD, requiring invasive procedures in the first 28 days of life. Fetal ultrasound often fails to adequately detect CCHD, discovering the ailment only 15%-50% of the time. The editorial concluded that "… surely the question now is not 'why should pulse oximetry screening be introduced?' but 'why should such screening not be introduced more widely?'" At the time of publication, The Lancet reported 16 states in the U.S. had either passed or introduced legislation for CCHD pulse oximetry screening.
CCHD screening gained wide exposure in a recent CNN news story, which describes the rationale for and benefits of pulse oximetry screening for CCHD detection. The piece includes clinical experts, product application examples using a SET pulse oximeter and sensor, and chronicles the devastating effect when a baby's CCHD is not detected at birth.
Joe Kiani, Founder and CEO of Masimo, stated: "It's time to screen every newborn. Screening with SET Pulse Oximetry has proven to not only save lives and families the unnecessary death due to CCHD, but it has been shown to be cost effective. Indeed, the best way to improve patient care and reduce cost of care is through medical innovations like these."
1 de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
2 Ewer AK, Middleton LJ, Furmston AT, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet 2011; 378: 785–94.
3 Meberg A, Andreassen A, Brunvand L, et al. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Pediatr 2009; 98: 682–86.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Study Affirms Masimo rainbow® Pulse CO-Oximetry™ Accuracy for Noninvasive Carboxyhemoglobin and Methemoglobin Measurements
Irvine, California – July 9, 2012 – Masimo (NASDAQ: MASI) announced today a new study in Respiratory Physiology & Neurobiology shows that noninvasive carboxyhemoglobin (SpCO®) and methemoglobin (SpMet®) measurements with Masimo rainbow® Pulse CO-Oximetry™ are accurate and conclude they can be used as an effective first screening test with emergency room patients suspected of suffering carbon monoxide (CO) poisoning.1
CO poisoning is a major cause of morbidity and mortality2 and is responsible for more than 50,000 emergency department visits per year in the United States.3 Because symptoms of CO poisoning are nonspecific – ranging from mild headache, nausea, confusion, and dizziness to end-organ injury, such as myocardial infarction, stroke, and death – diagnosis is difficult and has historically relied on clinical suspicion and confirmation by measurement of carboxyhemoglobin (COHb) via invasive blood-gas analysis. Unfortunately, it has been estimated that up to half of U.S. hospitals do not have invasive COHb testing ability – increasing the potential that many victims of CO poisoning could be overlooked and misdiagnosed.4
The study was conducted on healthy subjects who inhaled a mixture that included carbon monoxide such that it raised the COHb to 10% to 14%. Investigators compared the mean bias and precision of SpCO and SpMet data obtained noninvasively with a Masimo Rad-57™ Pulse CO-Oximeter and adult reusable SpCO rainbow® sensor (DCI-dc3) with invasive venous blood samples analyzed on an arterial blood-gas analyzer (ABL80 FLEX CO-oximeter, Radiometer America).
The SpMet results showed the mean bias was 0.0% and precision was 0.3%. The SpCO measurements showed a mean bias of –0.8% and precision of 2.5%.
Researchers noted the Rad-57 "provides coherent and reproducible day-to-day measurement" of SpCO and SpMet, and concluded the "Rad-57 should be used as a first screening to determine whether an invasive blood measurement of COHb should be performed to confirm the (CO) intoxication."
1 Zaouter C, Zavorsky G. "The measurement of carboxyhemoglobin and methemoglobin using a noninvasive pulse CO-oximeter." Respiratory Physiology & Neurobiology 2012 (http://dx.doi.org/10.1016/j.resp.2012.05.010)
2 Suner S, Patridge R, Sucov A, et al. "Noninvasive pulse CO-oximetry screening in the emergency department identifies occult carbon monoxide toxicity." J Emerg Med. 2008; 34(4):441-50.
3 Weaver LK. "Carbon monoxide poisoning." N Engl J Med. 2009; 360(12):1217-1225.
4 Hampson NB, Scott KL, Zmaeff JL. Carboxyhemoglobin measurement by hospitals: Implications for the diagnosis of carbon monoxide poisoning. J Emerg Med 2006;31(1):13-6.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


U.S. Medical Materiel Enterprise Standardization Office Selects Masimo Rad-57™ Handheld Pulse CO-Oximeter as Joint Product of Choice Rad-57s Available to All Branches of Military Medical Treatment Facilities and Field Units
Irvine, California – July 3, 2012 – The U.S. Department of Defense Military Health System has selected the Masimo Rad-57™ Handheld Pulse CO-Oximeter as the military's standardized handheld pulse oximeter for use worldwide, Masimo (NASDAQ: MASI) announced today. This agreement is available to all designated Medical TreatmentFacilities and Operational Field Units across the Department of Defense's global healthcare system, from first responders to definitive care.
The Defense Department selected the Rad-57 after product evaluations at 13 Medical Treatment Facilities worldwide and an extensive Joint Product Review Board selection process. The Rad-57 has already secured Air Worthiness Certification from the U.S. Army and Safe-to-Fly Certification from the U.S. Air Force.
The department initially will use the Rad-57 to measure oxygen saturation (SpO2) using Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry clinically proven in more than 100 independent and objective studies to provide accurate measurements even under the most challenging conditions. The Rad-57 also features Masimo rainbow® Pulse CO-Oximetry™, allowing clinicians to noninvasively monitor multiple blood constituents that previously required invasive or complicated procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and perfusion index (PI). Masimo rainbow® SET measurements are available with a simple software upgrade that the user may purchase at any time for the Rad-57.
Joe Kiani, Masimo CEO and Chairman of the Board, said, "The best place for our soldiers to be is safe at home. But when they are in harm's way, we want to be there for them. We are honored our life-saving technology will be with them to help them come home."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Signs Pronto-7™ Distribution Deal with PSS World Medical More U.S. clinicians to have access to the first and only FDA-cleared noninvasive spot-check hemoglobin, along with SpO2, PR, and PI monitor
Irvine, California – July 2, 2012 – Masimo (NASDAQ: MASI) announced today that PSS World Medical, Inc., (NASDAQ: PSSI) a leading national distributor of medical supplies and equipment to office-based physicians, will distribute the new Pronto-7™ – a palm-sized handheld device designed for quick and easy noninvasive spot-checking of total hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index.
 Pronto-7 will be available to PSS's U.S. customer base of physicians, skilled nursing and assisted living facilities, home health care and hospice agencies, and other alternate site healthcare providers in all 50 states.
Pronto-7 will be available to PSS's U.S. customer base of physicians, skilled nursing and assisted living facilities, home health care and hospice agencies, and other alternate site healthcare providers in all 50 states.
"We consider this product a significant breakthrough in patient management," said John Sasen, Chief Marketing Officer, PSS World Medical.
"We look forward to our relationship with Masimo introducing transformational offerings to our caregivers," added Eddie Dienes, President, PSS World Medical.
Masimo Pronto-7 has received notable industry accolades, including the 2011 GOLD Medical Design Excellence Award, 2011 TechAmerica High-Tech Innovation Award, and the 2011 iF Product Design Award.
The Pronto-7 offers a breakthrough solution for measuring hemoglobin in less than one minute – without the needles, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, pulse rate, and perfusion index, into any clinician's hands. The device's embedded 802.11 b/g and Bluetooth communication capability enables wireless printing or emailing of test results. Future upgrades will allow for wireless transmission to electronic health record (EHR) systems.
Masimo President of World Wide Sales, Marketing and Clinical Research, Jon Coleman, said: "PSS has a great reputation for bringing leading-edge medical technology to its customers. With the full market release of our breakthrough noninvasive hemoglobin monitoring products, we can leverage the sales footprint and customer relationships of PSS's professional field sales team to drive awareness of noninvasive hemoglobin technology and how doctors can use it to revolutionize their medical practices."
About PSS World Medical, Inc.
PSS World Medical, Inc. (Nasdaq: PSSI) markets and distributes medical products and services to front-line caregivers throughout the United States. With more than $1.5 billion in annual revenues and 4,000 team members, PSS is a leader in the markets it serves with innovative approaches to customer service and operational excellence. Its stated purpose is to strengthen the clinical success and financial health of caregivers by helping to solve their biggest problems. The Company is focused to accelerate growth in four markets - Physician, Laboratory, Dispensing, and Home Care & Hospice – with products and solutions that deliver high quality, cost effective, and convenient patient care.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Beth Graham
PSS World Medical, Inc.
(904) 380-4563
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Study Shows Masimo SET® Pulse Oximetry Outperforms Competing Technologies Masimo Radical® with SET Better than Nellcor N-600 with OxiMax, GE TruSat at Measuring SpO2 during Motion, Low Perfusion
Irvine, California – June 25, 2012 – Masimo (NASDAQ: MASI) announced today a new study in the Journal of Clinical Anesthesia demonstrates the Masimo Radical® with Masimo SET® pulse oximetry had higher oxygen saturation (SpO2) specificity and sensitivity and lower failure rates during conditions of motion and low perfusion than other "new-generation" pulse oximeters.1
Monitoring of SpO2 enables clinicians to detect hypoxemia (deficient blood oxygen) at an early stage. However, conventional pulse oximetry can fail during patient motion and low perfusion, triggering false alarms. More than 100 independent clinical studies have shown Masimo SET Measure-through Motion and Low Perfusion pulse oximetry has overcome the technological limitations of conventional pulse oximetry, and has made pulse oximetry accurate during challenging conditions, reducing false alarms and increasing true alarm detection.
In the study, conducted at the Long Beach Veterans Affairs Healthcare System in California, researchers evaluated 10 healthy volunteers and compared the Masimo Radical (SET V 5.0), the Nellcor N-600 (OxiMax V 1.1.2.0) and the GE TruSat (formerly Datex-Ohmeda) during motion and induced low perfusion. A total of 160 motion tests were performed, 80 with machine- and 80 with volunteer-generated motion. Researchers lowered room temperature to 16° C – 18° C (60.8F – 64.4F) to induce low perfusion. Sensitivity was calculated by dividing the number of tests in which the test device read <90% when the control device read <90% during hypoxia. Specificity was calculated by dividing the number of events that the test device reported SpO2 values >90% when the control device also reported values >90% during normoxia. Failure rate for each device was calculated by dividing the amount of time that each instrument displayed no SpO2 value by the total time of the test.
The study showed that during both machine- and volunteer-generated motion, Masimo SET had the highest SpO2 specificity (93% and 97%, respectively) and the highest SpO2 sensitivity (100% and 95%), while the Nellcor OxiMax had specificity of 67% and 77% and sensitivity of 65% and 50%, and the GE TruSat had 83% and 82% specificity and 20% and 15% sensitivity, respectively (p<0.05).
Masimo SET had failure rates of 0% during both machine and volunteer motions for SpO2. SpO2 failure rates during machine and volunteer motions were 9.3% and 16.4% for the Nellcor OxiMax and 1.3% and 1.7% for the GE TruSat (p
"This study reaffirms that Masimo SET delivers outstanding patient-monitoring, particularly in real-world conditions of patient motion and low perfusion," said Dr. Michael O'Reilly, Masimo's Chief Medical Officer. "We believe this performance difference is why clinicians are getting great results in different clinical scenarios, helping clinicians to reduce retinopathy of prematurity (ROP),2 screen for critical congenital heart disease (CCHD),3 and enabling continuous surveillance of patients in post-surgical wards.4 With fewer false alarms and greater true-alarm detection, Masimo SET offers clinicians and hospitals confidence that they are giving their patients the best care possible."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) http://www.sciencedirect.com/science/article/pii/S0952818012001481
2 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
3 Anne de-Wahl Granelli, Margareta Wennergren, Kenneth Sandberg, Mats Mellander, Carina Bejlum, Leif Inganäs, Monica Eriksson, Niklas Segerdahl, Annelie Agren, Britt-Marie Ekman-Joelsson, Jan Sunnegårdh, Mario Verdicchio, Ingegerd Ostman-Smith. "Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns." British Medical Journal (BMJ) January 2009; 338:a3037. http://www.bmj.com/content/338/bmj.a3037.full.
4 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Study Shows Masimo rainbow® Pulse CO-Oximetry™ Speeds Evaluation & Treatment of Carbon Monoxide Poisoning
Irvine, California – June 21, 2012 – Masimo (NASDAQ: MASI) announced today a new study in The American Journal of Emergency Medicine demonstrates for the first time that compared to invasive carboxyhemoglobin (COHb) measurement with laboratory CO-oximetry, noninvasive carboxyhemoglobin (SpCO®) measurement with Masimo rainbow® Pulse CO-Oximetry™ leads to more rapid diagnosis of carbon monoxide (CO) poisoning and a shorter time to the initiation of treatment with hyperbaric oxygen.1
Historically, CO levels in the blood have been measured using a laboratory CO-oximeter, requiring a patient or a patient's blood sample be transported to a hospital with laboratory CO-oximetry capability. In one region of the country, it was demonstrated that only one-half of acute care hospitals had laboratory CO-oximetry capabilities.2 Additional delays occur if a patient needs hyperbaric oxygen therapy, which often requires transfer to yet another medical center with hyperbaric capability.
In the study, based on three years of U.S. data from the Undersea Hyperbaric Medicine Society's CO poisoning surveillance system (supported by the Centers for Disease Control), researchers analyzed 1,711 cases of CO poisoning treated with hyperbaric oxygen. Of those, 1,606 had their initial carboxyhemoglobin (COHb) level measured by laboratory CO-oximetry; 105 with a Masimo Rad-57™ rainbow® Pulse CO-Oximeter. Of the 1,606 cases, 105 were selected to match the Pulse CO-Oximetry group on the basis of age, sex, race/ethnicity, intent of poisoning, and occurrence of loss of consciousness to compare the time it took to obtain a CO measurement, as well as the time from the end of CO exposure to treatment.
Patients who were initially measured using Pulse CO-Oximetry had a significantly shorter time to measurement of CO (1.1 vs. 1.7 hours, p<0.01), higher COHb levels, and a shorter period of time from the end of CO exposure to treatment (4.4 vs. 5.3 hours, p<0.01). Three hours after exposure, 45% of patients evaluated by Pulse CO-Oximetry had started treatment vs. just 25% of patients evaluated by laboratory CO-oximetry had started treatment (P< 0.01).
While previous studies have examined the accuracy of SpCO as well as the clinical utility of the Rad-57 for rapid mass screenings and detection of unsuspected CO poisoning, this is the first study Masimo is aware of to examine whether Pulse CO-Oximetry can help clinicians decrease the time patients are diagnosed and treated for CO poisoning.
Researchers concluded: "Patients evaluated with Pulse CO-Oximetry had significantly shorter times from CO exposure to COHb determination and hyperbaric oxygen treatment," adding, "it seems reasonable to consider further study of this simple and inexpensive technology for its potential benefit and to hope that even more time delay to treatment can be saved through increased use and adoption of standardized patient management algorithms."
1 Hampson N. "Noninvasive pulse CO-oximetry expedites evaluation and management of patients with carbon monoxide poisoning." The American Journal of Emergency Medicine 2012 (10.1016/j.ajem.2012.03.026) http://www.sciencedirect.com/science/article/pii/S0735675712001301
2 Hampson NB, Scott KL, Zmaeff JL. Carboxyhemoglobin measurement by hospitals: Implications for the diagnosis of carbon monoxide poisoning. J Emerg Med 2006;31(1):13-6.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Anesthesia Patient Safety Foundation Publishes Expanded Dartmouth Hitchcock Medical Center Results with Post-Surgical Monitoring Using Masimo SET® and Patient SafetyNet™ Opportunity cost savings on one floor alone estimated at $1.48 million per year
Irvine, California – June 18, 2012 – Masimo (NASDAQ: MASI) announced today that a follow-up analysis appearing in the Spring-Summer 2012 issue of the Anesthesia Patient Safety Foundation newsletter demonstrated that implementing Masimo SET Measure-through Motion and Low Perfusion pulse oximetry and the Masimo Patient SafetyNet™ remote monitoring and clinician notification system in all post-surgical, general floor units at Dartmouth Hitchcock Medical Center resulted in reduced rescue events and transfers to the intensive care unit (ICU) and offered significant opportunity cost savings.1 And even more impressive, no patients have died since the Masimo Patient SafetyNet system was instituted on the original study unit in December of 2007 as a result of respiratory depression from opioids.
The results confirm and expand the findings from the original 2010 landmark Anesthesiology publication of the study at Dartmouth Hitchcock showing that use of Masimo SET and Patient SafetyNet in a single orthopedic post-surgical unit led to a significant reduction in rapid response activations and intensive care unit (ICU) transfers.2 After the original study, Dartmouth Hitchcock mandated continuous monitoring for all patients in medical and surgical units when they are not directly observed by a healthcare provider.
After expanding post-surgical monitoring to the general and thoraco-vascular post-surgical units, Dartmouth Hitchcock reported:
- 57% overall reduction in rescue events over all units (4.4 to 1.9 per 1,000 patient days per month)
- 168 ICU days saved in the thoraco-vascular unit in the first 12 months after implementation – 10 more days per year than the original orthopedic unit
- 21% decrease in average length of stay of a patient with transfer to the ICU (total 5.1 days decreased, 1.8 days in the ICU and 3.3 days on the general floor) in the original orthopedic unit
- $1.48 million in annual opportunity cost savings in the original orthopedic unit due to the decreased ICU transfer rate (compared to initial costs just $167,993 for equipment and training and annual operational costs of just $58,261 for implementation and disposable sensors)
- $58,459 saved per patient who was not transferred to the ICU in the original orthopedic unit ($76,044 vs. $17,585)
Study authors Drs. Andreas H. Taenzer and George T. Blike stated: "Retrospective reviews demonstrated that adverse (patient) events are preceded by a period of physiologic instability of 6-8 hours.3,4 Therefore, identification of at-risk patients by spot checks every 6 hours for 10 minutes, which observes vital signs only 5% of the time, begs for improvement." They added, "While monitoring cannot prevent all physiologic deterioration, it can function as a 'patient safety airbag.'" The Dartmouth Hitchcock results speak for themselves, as the study authors reported that "no patients have suffered irreversible severe brain damage or died since patient surveillance was instituted on the original study unit in December of 2007 as a result of respiratory depression from opioids."
Dartmouth Hitchcock continues to investigate ways to further improve patient safety by expanding monitoring beyond pulse oximetry in higher risk populations such as post-operative patients on supplemental oxygen, in whom pulse oximetry can be a late indicator of respiratory depression. The study authors also reported their initial experience in evaluating Masimo rainbow® Acoustic Monitoring for noninvasive and continuous monitoring of respiration rate with a cloth sensor that is easily and comfortably applied to the patient's neck: "These (acoustic) monitors are better tolerated than our earlier trials in the immediate post-operative phase with chest straps for respiratory rate monitoring or nasal cannulas for end tidal CO2 monitoring, but not as well as finger pulse oximetry probes."
Joe Kiani, Masimo Founder and CEO, stated: "When we invented Masimo SET pulse oximetry over twenty years ago, we believed that by solving the motion artifact and low perfusion problem we could improve patient outcomes and reduce cost of care by taking noninvasive monitoring to new sites and applications, such as the general floor of hospitals. We applaud the exceptional work of the clinicians and investigators at Dartmouth Hitchcock Medical Center in showing the world that post-surgical monitoring with Masimo SET pulse oximetry and Patient SafetyNet do in fact help clinicians save lives and costs on the general floor. In today's healthcare climate, this is the perfect combination for hospitals under pressure to improve quality and reduce costs at the same time."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. http://www.apsf.org/newsletters/html/2012/spring/01_postop.htm
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Read more about Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study
3 Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. "Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital." Med J Aust 1999;171:22-5.
4 Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. "Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study." Resuscitation 2004;62:137-41.4
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Signs Pronto-7™ Distribution Deal with Henry Schein First & only FDA-cleared noninvasive spot-check hemoglobin monitor more widely available to clinicians nationwide
Irvine, California – June 13, 2012 – Masimo (NASDAQ: MASI) announced today that Henry Schein Medical, the Medical business of Henry Schein, Inc., (NASDAQ: HSIC), the world's largest provider of health care products and services to office-based practitioners, will distribute the new Pronto-7™ – a palm-sized handheld device designed for quick and easy noninvasive spot-checking of total hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index.
 The distribution agreement also covers Masimo's Pronto with rainbow® technology. Both the Pronto-7 and Pronto will be available to Henry Schein's U.S. customer base of physician offices, community health facilities, medical clinics, and ambulatory surgical centers.
The distribution agreement also covers Masimo's Pronto with rainbow® technology. Both the Pronto-7 and Pronto will be available to Henry Schein's U.S. customer base of physician offices, community health facilities, medical clinics, and ambulatory surgical centers.
"We are extremely pleased to be working with Masimo on the groundbreaking Pronto-7," said Dave McKinley, President, Henry Schein Medical. "Our customers rely on us to provide the latest technology for improving their practice and providing the highest quality care. We look forward to introducing our customers across the U.S. to this exciting, non-invasive innovation in patient care."
Masimo Pronto-7 has received notable industry accolades, including the 2011 GOLD Medical Design Excellence Award, 2011 TechAmerica High-Tech Innovation Award, and the 2011 iF Product Design Award. And in2010, noninvasive hemoglobin monitoring was named to the World Health Organization's (WHO) List of Innovative Medical Technologies that Address Global Health Concerns and Needs.
The Pronto-7 offers a breakthrough solution for measuring hemoglobin in less than one minute – without the needles risk of blood contamination, hazardous medical waste, laboratory analysis, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, pulse rate, and perfusion index, into any clinician's hands. The device's embedded 802.11 b/g and Bluetooth communication capability enables wireless printing or emailing of test results. Future upgrades will allow for wireless transmission to electronic health record (EHR) systems.
Masimo President of World Wide Sales, Marketing and clinical Research, Jon Coleman, said: "Henry Schein has a great reputation for bringing leading-edge medical technology to its customers. With the full market release of our breakthrough noninvasive hemoglobin monitoring products, we can leverage the sales footprint and customer relationships of more than 400 professional field sales professionals and 200 telesales professionals at Henry Schein Medical to drive awareness of noninvasive hemoglobin technology and how with it doctors can revolutionize their medical practice."
About Henry Schein, Inc.
Henry Schein, Inc. is the world's largest provider of health care products and services to office-based dental, medical and animal health practitioners. The Company also serves dental laboratories, government and institutional health care clinics, and other alternate care sites. A Fortune 500® Company and a member of the NASDAQ 100® Index, Henry Schein employs nearly 15,000 Team Schein Members and serves approximately 775,000 customers. The Company offers a comprehensive selection of products and services, including value-added solutions for operating efficient practices and delivering high-quality care. Henry Schein operates through a centralized and automated distribution network, with a selection of more than 90,000 national and Henry Schein private-brand products in stock, as well as more than 100,000 additional products available as special-order items. The Company also offers its customers exclusive, innovative technology solutions, including practice management software and e-commerce solutions, as well as a broad range of financial services. Headquartered in Melville, N.Y., Henry Schein has operations or affiliates in 25 countries. The Company's sales reached a record $8.5 billion in 2011, and have grown at a compound annual rate of 18% since Henry Schein became a public company in 1995. For more information, visit the Henry Schein Web site at www.henryschein.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Susan Vassallo
Henry Schein, Inc.
(631) 843-5562
[email protected]
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


United Kingdom's National Health Service Includes Masimo Pleth Variability Index® as "High Impact Innovation" for Intra Operative Fluid Management
Released as Part of Intra Operative Fluid Management Technologies Adoption Pack
Irvine, California – June 11, 2012 – Masimo (NASDAQ: MASI) announced today the National Health Service Technology Adoption Centre in the United Kingdom is advising hospitals to use Intraoperative Fluid Management Technologies as a way to improve patient outcomes, and included Masimo's noninvasive and continuous Pleth Variability Index (PVI®) among technologies available for helping clinicians manage fluid during surgeries.
"The use of Intraoperative Fluid Management Technologies (IOFMT) enables closer monitoring and management of patients' hydration status during major and high-risk surgery," the centre said in its Adoption Pack report. "Evidence shows that the use of this technology can facilitate improved outcomes for patients and benefits for the health economy."
The centre investigates, qualifies and supports new and innovative technologies that demonstrate benefits to patients and hospitals. The centre did not endorse one specific fluid-management tool. However, no other pulse oximetry technologies were included other than Masimo PVI.
Masimo PVI has been shown to help clinicians assess fluid responsiveness in adult surgical and intensive care patients under mechanical ventilation.1,2 Two studies have shown that PVI helps clinicians assess fluid responsiveness similarly to a more invasive and costly technology (Edwards Vigileo Monitor, Flo Trac catheter).3,4 Masimo PVI has also been shown to help clinicians improve fluid management to decrease patient risk in a randomized controlled trial.5
Although fluid administration is critical to optimizing patient status and enabling end organ preservation, unnecessary fluid administration is associated with increased morbidity and mortality.6 Traditional invasive measurements such as central venous pressure are not reliable to predict whether a patient will benefit from fluid administration. Newer advanced monitoring technologies that have been shown to help clinicians assess fluid responsiveness and improve fluid management are invasive, complex, and/or costly. In contrast, Masimo PVI is noninvasive and easy to use, and has no incremental procedural cost because pulse oximetry monitoring is already performed on all surgical and intensive care patients. Once Masimo SET pulse oximetry with PVI monitoring is available in a hospital, it can be considered in all patients in which an invasive arterial line or more complex or costly monitoring technologies may not be justified.
PVI is available on the Radical-7™ and Rad-87™ as well as select multiparameter patient monitors, such as Drager's Infinity® M540 and Welch Allyn's Connex® Vital Signs Monitor as part of Masimo rainbow® SET Pulse CO-Oximetry™ – the first-and-only technology platform to noninvasively measure blood constituents and fluid responsiveness that previously required invasive procedures, including: noninvasive and continuous total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and acoustic respiration rate (RRa™), in addition to the Measure-through Motion and Low-Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index.
Joe Kiani, Masimo's Founder and CEO said, "We applaud NHS' leadership in identifying processes and new technologies that can help advance medicine. Too many surgeries are performed throughout the world without assessing how patients will respond to fluid administration. The NHS's call for adoption of technologies to improve fluid management during surgeries is expected to improve patient outcomes. PVI is yet another innovation from Masimo that can play a major role in helping care providers around the world deliver better, more cost-effective care."
1 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia August 2008; 101(2):200-6. http://www.ncbi.nlm.nih.gov/pubmed/18522935.
2 Loupec T., Nanadoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically Ill Patients." Crit Care Med. 2011 Feb;39(2):294-9.
3 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology June 2010; 27(6):555-61. http://www.ncbi.nlm.nih.gov/pubmed/20035228.
4 Fu Q., Mi WD., Zhang H. "Stroke Volume Variation and Pleth Variability Index to Predict Fluid Responsiveness During Resection of Primary Retroperitoneal Tumors in Hans Chinese." BioScience Trends. 2012; 6(1):38-43 DOI: 10.5582/bst.2012.v6.1.38. http://www.ncbi.nlm.nih.gov/pubmed/22426102.
5 Forget P, Lois F, De Kock M. "Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesthesia & Analgesia. 2010 Oct;111(4):910-4..http://www.anesthesia-analgesia.org/content/early/2010/08/12/ANE.0b013e3181eb624f.abstract?ct=ct.
6 Bundgaard-Nielsen M et al. Acta Anaesthesiol Scand. 2007; 51(3):331-40
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo CEO Joe Kiani Named Ernst & Young Entrepreneur of the Year
Irvine, California – June 8, 2012 – Masimo (NASDAQ: MASI) CEO and founder Joe Kiani received the Ernst & Young (Orange County) Entrepreneur of the Year® Award during a gala at the St. Regis Monarch Beach Resort in Dana Point, Calif., Thursday evening.
Kiani is an inventor of pioneering noninvasive patient-monitoring devices, a trusted voice for patient safety and care, and a convention-breaking maverick.
Founded in 1989, Masimo has grown from a garage startup into a successful publicly traded company, employing more than 2,500 people worldwide. The company makes and markets Measure-through-Motion and Low Perfusion (blood flow) pulse oximetry technology such as Masimo rainbow® SET Pulse CO-Oximetry™ – a breakthrough platform that allows noninvasive, continuous monitoring of blood constituents and physiological parameters that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the Measure-through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
In 2006, Kiani and Masimo emerged victorious after a seven-year patent-infringement battle against a larger competitor. His underdog story is a testament to the power of the American Dream. The company filed for a successful initial public offering in 2007. Forbes named Masimo one of the best small public companies in the United States (November 2011). The company also has earned dozens of industry honors, including the 2011 TechAmerica High-Tech Innovation Award; the 2011 Gold Medal Medical Design Excellence Award; the 2011 iF Product Design Award; and multiple Zenith awards, among others.
In 2010, Kiani created the Masimo Foundation for Ethics, Innovation and Competition in Healthcare to encourage and promote activities, programs, and research opportunities that improve patient safety and deliver advanced healthcare to people worldwide who may not otherwise have access to lifesaving technologies.
"This is an honor and one that's made possible by the incredible, passionate and hard-working people at Masimo, who are motivated to help improve patient care and reduce healthcare costs," Kiani said of the Entrepreneur of the Year award. "We look forward to continuing to innovate, while taking noninvasive monitoring to new sites and applications."
About Ernst & Young Entrepreneur of the Year
Ernst & Young Entrepreneur of the Year is the world's most prestigious business award for entrepreneurs. The unique award makes a difference through the way it encourages entrepreneurial activity among those with potential, and recognizes the contribution of people who inspire others with their vision, leadership and achievement. As the first and only truly global award of its kind, Entrepreneur of the Year celebrates those who are building and leading successful, growing and dynamic businesses, recognizing them through regional, national and global awards programs in more than 140 cities in more than 50 countries.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Premier Healthcare Renews Supplier Agreement with Masimo Contract includes rainbow® SET Pulse CO-Oximetry™ and Masimo Patient SafetyNet™
Irvine, California – June 4, 2012 –Masimo (NASDAQ: MASI) announced today that it has been awarded a 33-month group purchasing renewal agreement with the Premier Healthcare Alliance, a leading national healthcare group purchasing organization. The new agreement allows Premier members, at their discretion, to take advantage of special pricing and terms pre-negotiated by Premier for Masimo's complete market-leading line of noninvasive products and solutions, including Masimo SET® pulse oximetry, rainbow® SET Pulse CO-Oximetry,™ rainbow® Acoustic Monitoring™, and Masimo Patient SafetyNet™.
Masimo President of World Wide Sales, Marketing and clinical Research, Jon Coleman, stated, "We are happy Premier recognizes that blood transfusions represent a large cost in a hospital and has committed to providing its membership with access to Masimo rainbow® SET platform that enables clinicians to noninvasively and continuously measure total hemoglobin (SpHb®), along with many other vital parameters, to help clinicians improve blood management and advance the standard of care."
Effective June 1, 2012, through February 1, 2015, Premier members can purchase Masimo Radical-7®, Rad-87®, and portable Rad-57® andPronto-7™ rainbow SET Pulse CO-Oximeters, and Masimo Patient SafetyNet™ on contract, along with ReSposable, Single Patient Use Adhesive, and Reusable sensors for all categories of patients (from neonatal and infant/pediatric to adult).
Masimo solutions available on contract with Premier include:
Masimo rainbow® SET Pulse CO-Oximetry
With Masimo rainbow® Pulse CO-Oximeters, clinicians can noninvasively and continuously measure many blood constituents that previously required invasive procedures, including: hemoglobin (SpHb), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to Measure-Through Motion and Low Perfusion Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate. And, as an upgradeable oximetry technology platform enabling new device capabilities to be added through simple field-installed software upgrades, Masimo rainbow® SET allows healthcare facilities to fight the rising costs of equipment obsolescence. Instead of costly device and hardware replacements, new noninvasive measurements and patient monitoring capabilities can be cost-effectively added to existing "rainbow-ready" Masimo oximeters via a quick, field-installed software upgrade tool.
Masimo Patient SafetyNet™ Remote Monitor Kennedy Health Systeming & Clinician Notification System
The Masimo SafetyNet remote monitoring and clinician notification system combines Masimo rainbow® SET Pulse CO-Oximetry and rainbow® Acoustic Monitoring with wireless clinician notifications via pager or smartphone – providing an unmatched level of safety for up to 80 patients on four floors. Masimo SafetyNet facilitates appropriate early clinical response to preempt sentinel events and avoid unnecessary ICU transfers while helping meet Joint Commission, APSF, and ASA guidelines for patient safety.
About the Premier healthcare alliance, Malcolm Baldrige National Quality Award recipient
Premier is a performance improvement alliance of more than 2,500 U.S. hospitals and 80,000-plus other healthcare sites using the power of collaboration to lead the transformation to high quality, cost-effective care. Owned by hospitals, health systems and other providers, Premier maintains the nation's most comprehensive repository of clinical, financial and outcomes information and operates a leading healthcare purchasing network. A world leader in helping deliver measurable improvements in care, Premier has worked with the Centers for Medicare & Medicaid Services and the United Kingdom's National Health Service North West to improve hospital performance. Headquartered in Charlotte, N.C., Premier also has an office in Washington. http://www.premierinc.com. Stay connected with Premier on Facebook, Twitter and YouTube
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the specificity and sensitivity of Masimo rainbow Pulse CO-Oximetry technology will provide continuous measurements of critical physiological parameters with real-time tracking and trending capabilities in all patients; risks related to the assumption that continuous Pulse CO-Oximetry measurements will enable clinicians to identify patient deterioration sooner and facilite earlier detection and treatment of potentially life-threatening conditions, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo 2012 Radical-7® and Pronto-7™ Shine at Nursing Expo Continuous and Spot-Check Pulse CO-Oximeters Take Star Turn at American Association of Critical Care Nurses' National Teaching Institute and Critical Care Exposition
Irvine, California – May 31, 2012 – Masimo (NASDAQ: MASI) showcased the 2012 Radical-7® and the Masimo Pronto-7™ at this year's American Association of Critical Care Nurses' National Teaching Institute and Critical Care Exposition in Orlando, Fla., May 22-24.
Kathleen Browning, a registered nurse at Mike O'Callaghan Federal Hospital in Nellis, Nevada, was among those who appreciated the 2012 Radical-7's wide-ranging functionality. "After a while, you can get a lot of machines at bedside," Browning said. "Combining them into one is a good idea. And it's noninvasive. If you're always drawing blood … then that can become a factor for the patient."
The 2012 wireless, touch screen, easily configurable Radical-7 upgradeable rainbow® SET platform features noninvasive and continuous monitoring of:
- Blood constituents that previously required invasive or complicated procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), pleth variability index (PVI®), carboxyhemoglobin (SpCO®), and methemoglobin (SpMet®)
- Masimo SET measure-through motion and low perfusion pulse oximetry for oxygen saturation (SpO2), pulse rate, and perfusion index
- Respiration rate through a novel acoustic sensor (RRa™) clinically shown to be as accurate as capnography for respiration rate.1
 Melissa Mulholland McAuslin, an RN in the post-anesthesia care unit at Mercy Hospital in Portland, Maine, saw the wireless, touch screen Pronto-7 as a possible remedy to her increasing workload.
Melissa Mulholland McAuslin, an RN in the post-anesthesia care unit at Mercy Hospital in Portland, Maine, saw the wireless, touch screen Pronto-7 as a possible remedy to her increasing workload.
"You have to do more, with less and do it faster," she said. "I particularly like the idea of a spot-check for hemoglobin. It would be such a big time-saver."
Hemoglobin is one of the most commonly ordered tests in both hospital and non-hospital settings because it is critical to assessing blood loss. The Pronto-7 offers a breakthrough solution for measuring hemoglobin in less than one minute – without the needles, risk of blood contamination, hazardous medical waste, and patient discomfort.
Tammy Green, an intensive care unit nurse at Beloit Memorial Hospital in Beloit, Wisc., said she is very familiar with the handheld Masimo Rad-57, which that hospital uses in its step down units to measure oxygen saturation, pulse rate and perfusion index.
She eagerly tested the Pronto-7, with an eye on the device's SpHb capability.
"That's so cool!" Green said, when the Pronto-7 indicated her hemoglobin level within moments. "I've never seen something like this," she added. "I could check it every day."
1 Ramsay MA, Lagow E, Usman M. Accuracy of Respiration Rate and Detection of Respiratory Pause by Acoustic Respiratory Monitoring in the PACU. NYPGA 2011 Abstract #P-9137; presented December 11, 2011
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Present at William Blair & Company 32nd Annual Growth Stock Conference
IRVINE, Calif., May 29, 2012 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the William Blair & Company 32nd Annual Growth Stock Conference at the Four Seasons Hotel in Chicago on Tuesday, June 12, 2012, at 1:30 p.m. Central Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statement
This press release includes forward-looking statements based on current expectations about future events affecting us, all of which are subject to risks and uncertainties, difficult to predict and could cause our actual results to differ materially and adversely. These include statements about our technology, focus and prospects, among others. Risks and uncertainties include those related to our technology, competition and intellectual property protection, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

First Published Study on Masimo Acoustic Respiration Rate Demonstrates Significantly Higher Patient Tolerance and Similar Accuracy Compared to Capnometry in Post-Surgical Patients
Irvine, California – May 17, 2012 – Masimo (NASDAQ: MASI) announced today a new study published in the May 2012 edition of the British Journal of Anaesthesia demonstrates that Masimo's acoustic respiration rate (RRa™) from rainbow® Acoustic Monitoring provides similar respiration rate accuracy as capnometry for extubated patients.1
Respiratory depression is common during early postoperative periods, especially after extubation (removal of the breathing tube) and when narcotic analgesics are required for pain management.2 Delayed detection of respiratory depression increases the risk of major neurological damage and death. Continuous monitoring of oxygenation and ventilation is recommended for all patients after general anesthesia.3 Capnometry is commonly used for intubated patients but in extubated patients, capnometry requires a nasal cannula or face mask that may be poorly tolerated or can be dislodged, leading to errors in data acquisition and false alarms.4,5
In the study, conducted at the University Hospital of Poitiers, France, researchers evaluated 52 post-surgical patients in the post-anesthesia care unit and compared the accuracy of RRa (Masimo Rad-87® with rainbow® Acoustic Sensors) and capnometry using a face mask (Oridion Capnostream 20™ with GHW Group Capnomask™). Patients were monitored for 16 to 60 minutes over a range of 6 to 24 breaths per minute, for a total of 99,002 data pairs. Differences in respiration rate of >4 bpm for 20 seconds or more between the two devices led to 28 clinical assessments of respiration rate, with 46% of errors attributed to the capnometer, 36% to RRa, and 18% to both methods. Events that negatively affected the accuracy of respiration rate included speaking (11 capnometry, 4 RRa), moving (6 capnometry, 1 RRa), and coughing (5 capnometry, 0 RRa). Comparing the bias of each subject between RRa and capnometry demonstrated limits of agreement for RRa of -1.4 to +1.4 breaths per minute.
Researchers concluded that, "monitoring postoperative respiration rate in extubated patients with an acoustic monitoring device is as accurate as capnometry through an adapted oxygen mask and may be a good alternative to time-consuming clinical assessment. The acoustic sensor was well tolerated while the face mask was removed by eight patients, leading to study discontinuation in two patients. The device appears to be well-tolerated and no more subject to error than capnometry."
Masimo's RRa allows clinicians to noninvasively and continuously assess patients' breathing – facilitating earlier detection of respiratory compromise and patient distress. RRa measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck.
1 Mimoz O, Benard T, Gaucher A, Frasca D, Debaene B. "Accuracy of Respiratory Rate Monitoring Using a Noninvasive Acoustic Method after General Anaesthesia." Br. J. Anaesth. May 2012; 108(5):872-875 http://www.ncbi.nlm.nih.gov/pubmed/22323525
2 Weiser TG, Regenbogen SE, Thompson KD, et al. "An Estimation of the Global Volume of Surgery: A Modeling Strategy Based on Available Data." Lancet 2008; 372:139-44.
3 "Practice guidelines for Postanesthetic Care: A Report by the American Society of Anesthesiologists Task Force on Postanesthetic Care." Anesthesiology March 2002; 96:742-52.
4 Miner JR, Heegaard W, Plummer D. "End-Tidal Carbon Dioxide Monitoring During Procedural Sedation." Acad Emerg Med 2002; 9:275-80.
5 Wilson J, Keeling P, Wright K, Woods J. "Thoraco-Abdominal Impedance Monitoring of Respiratory Rate During Sedation." Anaesthesia 2009; 64:1025-6.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Joins Summer Jobs+ Program, Offering Paid Internships, Free Workshops
Irvine, California – May 8, 2012 – Masimo (NASDAQ: MASI) today announced its participation in the White House's Summer Jobs+ program with a commitment to help up to 800 low-income or disconnected young people develop high-demand job skills through paid summer internships and a series of career-life workshops in 2012.
These opportunities align with Masimo's mission to help make a profound societal difference, with an emphasis on fostering cutting edge innovation and ethical conduct. Masimo's paid summer internship program will offer at least 10 young people meaningful opportunities to develop marketing, engineering and other skills and serve as a solid career stepping stone.
Masimo also is partnering with community groups and nonprofit organizations to provide intensive, engaging and enlightening workshops to help hundreds of students and underserved youth gain crucial work-life strategies to help them succeed, regardless of their career path.
The federal government and private sector in January committed to creating nearly 180,000 employment opportunities for low-income youth in the summer of 2012. As pathways to careers, summer employment is critical to the success of young people, good for business, and important for our country. Today's youth are struggling to get work experience they need for the jobs of the future. Last summer, the unemployment rate among youth ages 16-24 set a near record high, and only 21 out of 100 low-income teens had a job.
Joe Kiani, Masimo CEO and Chairman of the Board, said, "The Summer Jobs+ is a great program and demonstrates what can be accomplished when government, nonprofits and the private sector band together to fix real problems. Masimo is proud to do its part today to help prepare the workforce of tomorrow. There's still time for more entities to join. We hope more will so that we can get our youth inspired to build our future."
Companies and nonprofits interested in participating can visit the Summer Jobs+ website; young people interested in jobs, internships and other opportunities can visit the new Summer Jobs+ Bank.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Ospedale Valduce and Masimo Announce Italy's First Installation of Masimo Patient SafetyNet™
Irvine, California – May 8, 2012 – Ospedale Valduce and Masimo (NASDAQ: MASI) today announced Italy's first pediatric installation of Masimo Patient SafetyNet™ and Radical-7® bedside devices with oxygenation and pulse rate monitoring with Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry.
"SafetyNet provides hospitals with financial benefits by improving efficiency in patient flow, reducing rapid response calls, better utilizing NICU bed resources, and reducing patient length of stay," said Dr. Daniele Merazzi, chief of Valduce's neonatal intensive care unit. "With Masimo's SafetyNet, alarms are instantly sent to assigned clinicians through a flexible, cost-effective system that can leverage existing IT infrastructure, resulting in documented patient-safety improvements."
SafetyNet helps keep patients safer by noninvasively, continuously measuring and tracking the underlying physiological conditions of up to 80 patients on four floors. SafetyNet detects real-time changes and abnormalities that signal declining health status. When a patient's condition deteriorates, the system automatically sends wireless alerts directly to the pager or cellphone of assigned clinicians – prompting a potentially lifesaving response at the patient's bedside.
SafetyNet has been clinically proven to significantly decrease rapid response activations by 65% and costly ICU transfers by 48% to improve patient outcomes and reduce costs.1 Masimo Radical-7s feature Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, proven in more than 100 independent and objective studies to provide accurate and reliable oxygen saturation measurements during motion and low perfusion.
Jon Coleman, President of Worldwide Sales, Marketing, & Clinical Research, stated, "We are happy to support Ospedale Valduce provide the best care available for its most vulnerable patients. This is another great example of how Masimo technology continues to advance patient safety around the world."
1 Taenzer AH, Pyke JB, McGrath SP, Blike GT. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study" Anesthesiology; February 2010; Vol 112, Issue 2, pp 282-287.
About Ospedale Valduce
Valduce is a private religious hospital founded in 1853 by Mother Giovannina Franchi near Lake Como in Northern Italy. In 1974 Valduce became a "classified hospital." The 290-bed facility now includes anesthesia, cardiology, general surgery and pediatric departments. For more information, visit http://www.valduce.it/h_ospedale.htm
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Pier Luigi Bonini
Ospedale Valduce
Phone: (+ 39) 031324111
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Novation Inks Supplier Contract Category Agreement for Masimo SET® pulse oximetry and rainbow® SET Pulse CO-Oximetry™
Irvine, California – May 3, 2012 – Masimo (NASDAQ: MASI) today announced it has signed a three-year, dual-source agreement with Novation, the healthcare contracting services company of VHA Inc. and the University Health System Consortium (UHC). The agreement, effective May 1, 2012, covers Masimo SET® pulse oximetry and Masimo rainbow® SET Pulse CO-Oximetry™ and rainbow® Acoustic Monitoring, including standalone monitoring devices, handhelds, and sensors. The competitive bid process involved an extensive clinical review and a technology value analysis by Novation's member-based pulse oximetry task force.
Masimo SET has been clinically proven in over 100 independent studies to provide the most accurate and reliable SpO2 and pulse rate measurements, even under the most challenging conditions of patient motion and low peripheral perfusion. Utilizing patented signal processing technologies, including parallel engines and adaptive filters, Masimo SET delivers accurate and reliable measurements of a patient's true oxygenation status when conventional pulse oximetry technologies don't – shown to perform at 95% false alarm sensitivity and 97% true alarm sensitivity.1
Masimo rainbow® SET Platform
Masimo rainbow SET® Pulse CO-Oximetry™, a breakthrough technology platform featuring the accuracy and reliability of Masimo SET, is continuing to revolutionize patient monitoring by significantly expanding the ability to noninvasively capture, continuously track and monitor multiple blood constituents, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to Measure-through Motion and Low Perfusion performance of Masimo SET oxyhemoglobin (SpO2), and pulse rate (PR).
Masimo Patient SafetyNet™ Remote Monitoring & Clinician Notification System
The Masimo Patient SafetyNet remote monitoring and clinician notification system combines Masimo rainbow SET Pulse CO-Oximetry™ and rainbow® Acoustic Monitoring with wireless clinician notifications via pager or smartphone – providing an unmatched level of safety for up to 80 patients on four floors. Patient SafetyNet facilitates appropriate early clinical response to preempt sentinel events and avoid unnecessary ICU transfers while helping meet Joint Commission, APSF, and ASA guidelines for patient safety.
"We are happy to announce this new agreement with Novation," said Jon Coleman, Masimo's President of World Wide Sales and Marketing. "Since being added to the Novation contract in 2003, our annualized sales to Novation hospitals has increased by over 4,000%, signifying the demand for our technology. Through this agreement, VHA and UHC members have access to Masimo SET pulse oximetry products and rainbow SET technology that may allow clinicians to detect and treat an expanding number of potentially life-threatening conditions."
1 Shah N et al. Anesthesiology. 2006;105:A929. (abstract)
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Masimo Reports First Quarter 2012 Financial Results
Q1 2012 Highlights (compared to Q1 2011):
- Product revenue rose 11% to $112.2 million
- Masimo rainbow revenue rose 14% to $8.5 million, including 70% increase in SpHb revenue
- Shipped 33,300 Masimo SET® and Masimo rainbow® SET units
- Net income was $15.8 million, with EPS of $0.27
Irvine, California, May 2, 2012 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended March 31, 2012.
Masimo's total first quarter revenue, including royalties, rose 6% to $119.2 million, compared to $113.0 million for the first quarter of 2011. First quarter 2012 product revenue rose 11% to $112.2 million, compared to $101.6 million for the first quarter of 2011. The company's worldwide end-user business grew 12% in the first quarter of 2012 and represented 85% of product revenue. OEM sales, which represented the remaining 15% of product revenue in the first quarter, were flat compared to the same period in 2011. Revenue from Masimo rainbow product sales rose 14% to $8.5 million in the first quarter, compared to $7.4 million for the first quarter of 2011. First quarter 2012 rainbow revenue included a 70% increase in total hemoglobin (SpHb) sales, compared to the first quarter of 2011.
Net income for the first quarter was $15.8 million, or $0.27 per diluted share, compared to net income of $18.0 million, or $0.30 per diluted share, in the first quarter of 2011. The decline reflects a $4.5 million reduction in royalty revenue received from Covidien due to a decline in the royalty rate, effective March 15, 2011.
During the first quarter, the company shipped approximately 33,300 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, compared to approximately 43,100 in the same prior-year period. Masimo estimates its worldwide installed base as of March 31, 2012 to be 1,005,000 units, up 13% from 890,000 units as of April 2, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo delivered a solid start to 2012 with 11% product revenue growth, fueled by double-digit growth in both our U.S. acute care and international direct channels, coupled with a 14% rise in global rainbow sales. In addition, our worldwide installed base exceeded one million units for the first time ever, signaling continued increased demand for our breakthrough noninvasive monitoring technologies that benefit hospitals, clinicians and patients by improving care and reducing costs."
As of March 31, 2012, cash and cash equivalents were $128.8 million, compared to $129.9 million as of December 31, 2011. The change reflects primarily net cash generated from operations, offset by $7.2 million in cash used to purchase the assets of Spire Semiconductor and $14.4 million in cash used to repurchase shares of Masimo common stock. Including shares repurchased early in the second quarter of 2012, the company completed its full three million share repurchase program authorized by the Board of Directors in August 2011.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 69297005. After the live webcast, the call will be available on Masimo's website through June 1, 2012. In addition, a telephonic replay of the call will be available through May 15, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 69297005.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Media Contact:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
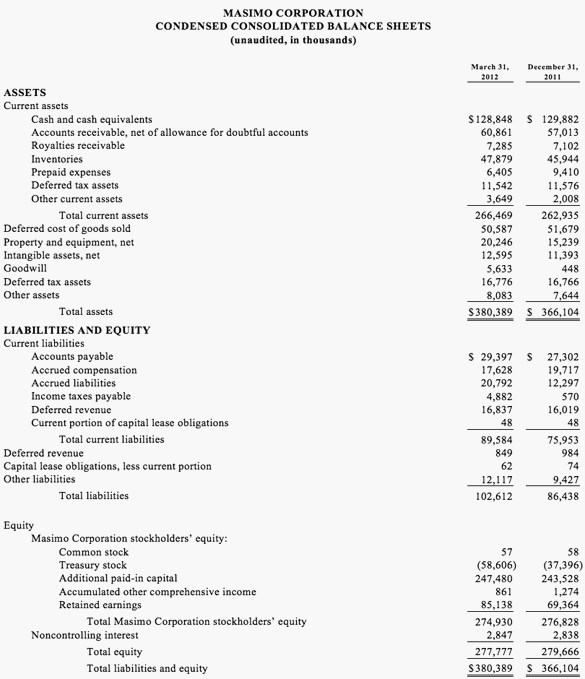




Masimo to Present at Deutsche Bank Securities 37th Annual Health Care Conference
IRVINE, Calif., April 26, 2012 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Deutsche Bank Securities 37th Annual Health Care Conference at the InterContinental in Boston on Wednesday, May 9, 2012, at 8 a.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statement
This press release includes forward-looking statements based on current expectations about future events affecting us, all of which are subject to risks and uncertainties, difficult to predict and could cause our actual results to differ materially and adversely. These include statements about our technology, focus and prospects, among others. Risks and uncertainties include those related to our technology, competition and intellectual property protection, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces New Low-Profile Infant and Neonatal Pulse Oximetry Sensors Smaller, Flexible Sensors Are More Comfortable for Patients, Easier to Apply for Clinicians
Irvine, California – April 25, 2012 – Masimo (NASDAQ: MASI) today announced the full market release of low-profile sensors to monitor oxygen saturation in newborns. The newly enhanced low-profile LNCS®, M-LNCS Neo®, NeoPt®, and Inf Sensors® are smaller and thinner, making them significantly more comfortable for patients and easier to apply for healthcare workers.
Masimo's breakthrough infant and neonatal sensor enhancements offer stellar performance, reliability, and accuracy, as well as compatibility with Masimo's noninvasive SET® and rainbow® SET technologies, which continuously monitor oxygenation, pulse rate, and blood constituent measurements that previously required invasive or complicated procedures.
"Masimo's new low-profile sensors are not only flatter and easier to apply, but the added pliability allows them to fit around the extremities better, so they stay on better," said Elizabeth Berube, patient care coordinator with University Health System-San Antonio, a 498-bed hospital. "When a baby's only a pound and a half at birth, it makes a difference to have this little, more streamlined sensor."
Masimo's breakthrough low-profile sensor components perform to the highest standards clinicians the world over have come to expect from Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry.
Blood-oxygen monitoring plays a key role in maintaining the health of newborns. Too little oxygen can lead to brain damage, organ failure or death; too much oxygen can cause blindness. A multi-center study published in the international peer-reviewed academic journal Acta Paediatrica showed that a change in clinical practice with the use of Masimo SET pulse oximetry technology led to a significant reduction of severe retinopathy of prematurity (ROP) – a devastating eye disorder resulting in partial or complete blindness in premature newborns. The study also confirmed that conventional pulse oximetry technology is not effective in reducing ROP, even when clinical practice is changed to reflect lowered oxygen saturation targets.1
Joe Kiani, Masimo CEO and Chairman of the Board, stated: "The release of our new line of low-profile sensors will enable clinicians to better care for their newborn patients, and offer the most vulnerable of our population a greater chance of leading healthy lives. We're proud to deliver another industry first – a line of low-profile, extremely small and highly effective sensors for healthcare providers worldwide."
1Castillo A., Deulofeut R., Critz A., Sola A. "Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology." Acta Paediatrica.2011 Feb; 100 (2): 188–192.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Essential Medical Dismisses Patent Case against Masimo and Cercacor
Irvine, California – April 23, 2012 – Masimo (NASDAQ: MASI) , the inventor of rainbow® Pulse CO-Oximetry™, rainbow® Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced that Essential Medical Devices, Inc. dismissed all outstanding claims against Masimo in the patent case it had filed against Masimo and Cercacor. Masimo did not incur any liability to Essential Medical, and the dismissal included a covenant not to sue on the asserted patent.
On August 19, 2011, Essential Medical had filed a patent infringement lawsuit against Masimo and Cercacor, alleging infringement by Masimo's Rainbow technology.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI is capable of detecting fluid responsiveness in virtually all ICU patients, and risks related to our assumptions that Masimo PVI monitoring has the potential to decrease hospital stay, mechanical ventilation, postoperative morbidity, and costs in patients undergoing high-risk surgery, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Careby Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Masimo SafetyNet and PSN are under license from University HealthSystem Consortium.


New Study Shows Masimo's Noninvasive Pleth Variability Index Provides Similar Ability to Assess Fluid Responsiveness During Surgery as More Costly and Invasive Method
Irvine, California – April 19, 2012 – Masimo (NASDAQ: MASI) announced today that a study published in the current issue of peer-reviewed journal BioScience Trends demonstrates that noninvasive and continuous monitoring of Masimo Pleth Variability Index (PVI®) helps clinicians assess fluid responsiveness during major abdominal surgery and concluded that PVI results were similar to invasive, more expensive stroke volume variation (SVV).1 Other traditional hemodynamic variables were not significant for assessing fluid responsiveness.
In the study of 51 patients, 31 patients were responders to fluid administration and 20 were non-responders, defined as a >10% increase stroke volume index (the amount of blood a heart pumps in each beat) after fluid administration. PVI was measured from a Masimo Radical-7 and LNOP Adt and SVV was measured from a Vigileo® device and Flotrac® catheter. A PVI threshold of >13.5% discriminated responders with a sensitivity of 77% and a specificity of 81%, while an SVV threshold of >12.5% discriminated responders with a sensitivity of 87.9% and a specificity of 83.3%. The areas under the curve (AUC) for fluid prediction for PVI and SVV were 0.79 and 0.86 respectively, while the AUCs for stroke volume index, cardiac index, central venous pressure, and mean arterial pressure were not statistically significant.
"There was no significant difference between the areas under the receiver operating characteristic curve for SVV and PVI," the study's authors stated. "Our results showed that SVV and PVI could predict fluid responsiveness during major abdominal surgery in complicated dynamic conditions" and concluded that "monitoring fluid responsiveness using a noninvasive device may help for fluid optimization in the operating room, especially in some patients who do not need invasive artery monitoring."
Multiple studies have shown PVI helps clinicians assess fluid responsiveness in surgical, mechanically ventilated patients – helping clinicians improve fluid management to reduce patient risk2,3. Although fluid administration is critical to optimizing patient status and enabling end organ preservation, unnecessary fluid administration is associated with increased morbidity and mortality. Because SVV has been shown in previous studies to be an accurate method to predict fluid responsiveness and optimize fluid management in surgical and intensive care patients, the similar accuracy shown with noninvasive PVI suggests that PVI-guided fluid optimization may also help guide fluid management to minimize patient risk, as was already shown in one randomized controlled trial.4
Masimo Chief Medical Officer Dr. Michael O'Reilly said, "There is tremendous variability among providers in the amount of fluid they administer for a given procedure or a given patient. Too little fluid can lead to under resuscitation and decreased tissue perfusion and decreased organ function. Too much fluid can lead to over-resuscitation, which can lead to cardiac failure and pulmonary edema. Moreover, too much fluid complicates the job of the surgeon by making anastomosis and closures trickier. Fluid responsiveness assessment and goal directed therapy are growing requirements to advance patient safety. The vast majority of surgeries performed in the world today are performed without assessing how a patient will respond to fluid administration, leading to sub-optimal resuscitation. Masimo PVI is a safe, reliable, and noninvasive way to guide fluid management to minimize patient risk."
1 Fu Q., Mi WD., Zhang H. "Stroke Volume Variation and Pleth Variability Index to Predict Fluid Responsiveness During Resection of Primary Retroperitoneal Tumors in Hans Chinese." BioScience Trends. 2012; 6(1):38-43 DOI: 10.5582/bst.2012.v6.1.38. http://www.ncbi.nlm.nih.gov/pubmed/22426102.
2 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia August 2008; 101(2):200-6. http://www.ncbi.nlm.nih.gov/pubmed/18522935.
3 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology June 2010; 27(6):555-61. http://www.ncbi.nlm.nih.gov/pubmed/20035228.
4 Forget P, Lois F, De Kock M. "Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesthesia & Analgesia. 2010 Oct;111(4):910-4.. http://www.anesthesia-analgesia.org/content/early/2010/08/12/ANE.0b013e3181eb624f.abstract?ct=ct.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI is capable of detecting fluid responsiveness in virtually all ICU patients, and risks related to our assumptions that Masimo PVI monitoring has the potential to decrease hospital stay, mechanical ventilation, postoperative morbidity, and costs in patients undergoing high-risk surgery, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Careby Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Masimo SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report First Quarter 2012 Financial Results after Market Close on May 2
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, California – April 18, 2012 – Masimo (NASDAQ: MASI) announced today that it will release first quarter financial results for the period ended March 31, 2012, after the market closes on May 2, 2012. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 69297005. After the live webcast, the call will be available on Masimo's website through June 2, 2012. In addition, a telephonic replay of the call will be available through May 16, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 69297005.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
(949) 297-7043
[email protected]
Media Contact:
Mike Drummond
[email protected]
(949) 297-7434
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Pronto-7™ Receives China and South Korea Regulatory Clearances
Irvine, California – April 12, 2012 – Masimo (NASDAQ: MASI) announced the China State Food and Drug Administration and South Korea Food and Drug Administration regulatory clearances of Masimo Pronto-7™ – enabling clinicians throughout both countries to conveniently and noninvasively measure total hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index.
 Earlier this year, Masimo announced U.S. Food and Drug Administration 510(k) clearance and full market release of the new Pronto-7, which includes expanded sensor size options to accommodate a wider range of finger sizes and the addition of a Max Sensitivity Mode.
Earlier this year, Masimo announced U.S. Food and Drug Administration 510(k) clearance and full market release of the new Pronto-7, which includes expanded sensor size options to accommodate a wider range of finger sizes and the addition of a Max Sensitivity Mode.
Su Lei, director of ICU at China's General Hospital of Guangzhou Military Command, praised the Pronto-7 as a time- and potentially life-saving device. "In our ICU, Pronto-7 helps to get the SpHb value in an extremely quick way," Lei said. "The Pronto-7 quickly determines the hemoglobin levels for acute and critically ill patients and helps our staff with early intervention when necessary."
The Pronto-7 measures hemoglobin in less than one minute – without the needles, risk of blood contamination, hazardous medical waste, including blood contaminated sharps, laboratory analysis, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, pulse rate, and perfusion index, into any clinician's hands. The device's embedded 802.11 b/g and Bluetooth® communication capability offers wireless printing or emailing of test results. Future upgrades will allow for wireless transmission to electronic health record (EHR) systems.
Masimo Founder and CEO, Joe Kiani, stated: "Regulatory clearance of the Pronto-7 in China and South Korea will offer clinicians in those countries better ways to monitor and care for their patients. We are proud our innovative, completely noninvasive technology will enable clinicians to deliver the right care, at the right time to patients in these countries."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Careby Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Fortis Healthcare Signs Agreement Giving India's Fastest-Growing Hospital Chain
Access to Masimo's State-of-the-Art Noninvasive Patient Monitoring Solutions
Fortis Escorts Heart Institute in New Delhi is First to Standardize Operating Rooms and Critical Care Areas to Masimo rainbow® SET Pulse CO-Oximetry Technology for Advanced Noninvasive Measurements
Irvine, California – April 2, 2012 – Fortis Healthcare, India's leading provider of integrated healthcare services with 68 hospitals across the country, and Masimo (NASDAQ: MASI) jointly announce a multi-year medical technology supplier agreement that allows Fortis hospitals access to Masimo's full line of the most technologically and clinically-advanced pulse oximetry and noninvasive, continuous patient monitoring solutions.
As part of the new agreement, Fortis Escorts Heart Institute, New Delhi, becomes the first hospital in the Fortis Healthcare Group to install 100 Masimo Radical-7s to standardize its operating rooms and critical care patient rooms with Masimo technology.
"This new preferred supplier relationship enables all hospitals and healthcare facilities owned, managed, and contracted with Fortis Healthcare to directly access and purchase Masimo's complete market-leading line of advanced noninvasive patient monitoring solutions," stated Dr. Raajiv Singhal, zonal director, Fortis Healthcare. "Now all Fortis-affiliated hospitals, beginning with Fortis Escorts Heart Institute, can benefit from the advanced noninvasive capabilities of Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, Masimo rainbow® SET Pulse CO-Oximetry™, Masimo SafetyNet™, and SEDLine® brain function monitoring solutions at significant contracted savings."
Fortis hospitals now can purchase Masimo standalone (Radical-7®, Rad-87®, Rad-8®) and portable (Rad-5®, Rad-57®, Pronto, Pronto-7™) rainbow Pulse CO-Oximeters on contract, along with ReSposable, adhesive, and reusable sensors for all categories of patients, from neonatal and infant/pediatric to adult.
Dr. Anil Karlekar, head of anesthesia and critical care medicine at Fortis Escorts Heart Institute, stated, "This long-term association with Masimo will provide Fortis with industry-leading medical technology innovations and patient-monitoring solutions that will benefit patient care and the Fortis vision of creating a world-class integrated healthcare delivery system in India."
As the first hospital in the Fortis network to standardize its operating rooms and critical care areas to Masimo rainbow SET Pulse CO-Oximetry technology, Fortis Escorts Heart Institute recently installed 100 Masimo Radical-7s – with a select number providing clinicians with access to Masimo noninvasive total hemoglobin (SpHb®) and pleth variability index (PVI®) measurement capabilities. Monitoring SpHb helps clinicians quickly and continually assess a patient's hemoglobin blood level, which may help reduce unnecessary blood transfusions and associated costs. PVI may allow clinicians to noninvasively and continuously assess fluid status of patients. Masimo noninvasive oximeters and sensors enable rapid and continuous blood-constituent monitoring without the need to draw blood – providing tools to detect signs of anemia, internal bleeding, and the need for a blood transfusion in patients undergoing surgery. And because the same sensors follow patients from the OR to critical care areas of the hospital, each patient has a lifesaving continuum of care.
"Since installing the new technology in our operating rooms, Masimo Radical-7 monitors have noninvasively tracked and trended heart rate, oxygen saturation, hemoglobin, and fluid volume changes reliably," Dr. Karlekar added, "providing a unique new view into the patient's underlying physiological status, which has the potential to improve clinical decisions during surgery and patient outcomes during recovery."
Masimo rainbow SET Pulse CO-Oximetry is a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures – including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry at leading hospitals worldwide, Masimo rainbow SET provides real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.*
Masimo rainbow Pulse CO-Oximeters deliver additional features with simple field-installed software upgrades, so healthcare facilities can fight the rising costs of equipment obsolescence. No more costly device and hardware replacements. Masimo's easy upgrader tool adds new noninvasive measurements and patient-monitoring capabilities to existing "rainbow-ready" Masimo oximeters.
Masimo President of Worldwide Sales, Marketing, and Clinical Research, Jon Coleman, stated, "Fortis Healthcare is blazing a healthcare transformation trail in India with Masimo SET and rainbow SET advanced noninvasive technologies. Leveraging the unique benefits of our noninvasive oximetry and patient monitoring measurements and devices provides Fortis clinicians with immediate access to clinical measurements and critical physiologic intelligence, like real-time hemoglobin levels, that previously required invasive, time-consuming, and costly testing with delayed results. This translates to potentially earlier clinical decision-making that improves patient safety and outcomes while reducing the cost of care."
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: http://www.masimo.com/cpub/clinicals.htm.
About Fortis Healthcare
Fortis Healthcare (India) Limited is committed to clinical excellence and patient-centric healthcare, which is manifest in hospital design, patient services, medical programs and the compassionate approach of medical and non-medical hospital staff. Fortis commissioned its first hospital in 2001 at Mohali, near Chandigarh, and has expanded its operations to become a network with an over 10,000 bed capacity across 68 hospitals. The Fortis network has a large number of international accreditations, including four JCI (Joint Commission International) and 10 NABH (National Accreditation Board for Hospitals) certifications. The network's capability covers multi-speciality hospitals and super-speciality centres that provide tertiary and quaternary healthcare to patients in the major medical specialities. For more information, visit www.fortishealthcare.com
About Fortis Escorts Heart Institute
Recognized the world over as a center of cardiac excellence, Fortis Escorts Heart Institute provides the latest technology in cardiac bypass surgery, minimally invasive surgery, interventional cardiology, noninvasive cardiology, and pediatric cardiology to more than 14,500 inpatients and 7,200 emergency patients a year. With more than 200 cardiac doctors, 1600 employees, 285 patient beds, 5 Cath Labs, and a host of other world-class facilities, Fortis Escorts Heart Institute is backed by the most advanced laboratories and the latest technology for cardiac care.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Ruhee Dhar
Fortis Healthcare
Phone: 91-11-47135000
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Debuts 2012 Radical-7® at World Congress of Anaesthesiologists Breakthrough Functionality Automates the Process of Care and Enables Clinician-Centric Monitoring
Irvine, California – March 26, 2012 – Masimo (NASDAQ: MASI), the inventor of rainbow Pulse CO-Oximetry™, rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, debuted its 2012 Radical-7 monitor at the 15th World Congress of Anaesthesiologists conference in Buenos Aires, Argentina, March 25-30, 2012.
- Automatic rotational screen for either
horizontal or vertical display
Once again raising the technologic bar, the 2012 Radical-7 leverages Masimo's breakthrough noninvasive measurements with breakthrough functionality designed to automate the process of care and enable clinicians to instantly adapt to changing monitoring needs in individual patients and care areas.
The 2012 Radical-7's upgradeable rainbow® SET platform features noninvasive and continuous monitoring of:
- Blood constituents that previously required invasive or complicated procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), pleth variability index (PVI®), carboxyhemoglobin (SpCO®), and methemoglobin (SpMet®)
- Masimo SET measure-through motion and low perfusion pulse oximetry for oxygen saturation (SpO2), pulse rate, and perfusion index
- Respiration rate through a novel acoustic sensor (RRa™) clinically shown to be as accurate if not more than capnography for respiration rate, or even through the plethysmograph waveform (RRp™), though less reliable than RRa.
-
-
Detachable, battery-operated, wireless handheld or wearable device to facilitate untethered monitoring during transport and ambulation with built-in wi-fi.
-
The 2012 Radical-7 is rainbow® centric, with its intuitive, gesture-control, touch screen that allows SpHb, SpOC, SpCO, SpMet, and RRa to be viewed as prominently as SpO2 and pulse rate. The 2012 Radical-7 offers exceptionally easy operation and instant adaptability to change displays and settings on the fly. With a quick touch, drag, and drop, clinicians can move any parameter to and from center and bottom of the display, never losing track of any vital signs. The instant-trend feature provides the ability to view one or two parameters at once and with a simple finger gesture, clinicians can move, expand, or collapse parameter trends for real-time analysis. Standard wireless connectivity from integrated 802.11 and Bluetooth® technology in the handheld Radical-7 keeps patients connected even while they are ambulating to enable clinician notification and automate charting and enable meaningful use of electronic health record systems.
The 2012 Radical-7 includes a variety of features designed to simplify the clinician's life. Adaptive Threshold Alarm™, helps clinicians manage nuisance alarms and reduces the time required to set patient-specific alarms by automatically adjusting the audible alarm to the patient's baseline. In Vivo Adjustment™, allows clinicians to adjust the noninvasive measurements to the specific patient and laboratory reference device they use for invasive blood testing. These capabilities received the CE Mark in 2011 and are pending FDA 510(k) clearance.
The Radical-7 offers unprecedented versatility in multiple care areas with its automatic rotational screen for either horizontal or vertical display and three-in-one capability to be used as: 1) a standalone device for bedside monitoring; 2) a detachable, battery-operated wireless device handheld or wearable device to facilitate untethered monitoring during transport and ambulation; and 3) a multiparameter monitoring interface via SatShare®, allowing hospitals to seamlessly implement rainbow® measurement capabilities in the Radical-7 display while automatically sending the Radical-7's Masimo SET pulse oximetry measurements for display on the multiparameter monitor.

- Intuitive, gesture-control, touchscreen for exceptionally easy operation and instantly adaptable functionality.
The 2012 Radical-7 continues to provide respiration rate with unprecedented ease and reliability via revolutionary rainbow Acoustic Monitoring™. The Radical-7 now also includes the ability to measure respiration rate from the plethysmograph waveform (RRp™), enabling monitoring of breathing status from a standard Masimo SET pulse oximetry or rainbow® Pulse CO-Oximetry sensor in all patients in whom those sensors are indicated. The RRp measurement received the CE Mark in March 2011 and has been available on the Rad-87® monitor, but this is the first time it is available on the Radical-7 in CE Mark countries. The RRp measurement is determined by the variations in the plethysmograph waveform due to respiration, although the measurement is not possible in all patients or conditions. For patients in whom reliable and continuous respiration rate monitoring is required, Masimo's acoustic respiration rate (RRa) measurement provides accurate and patient-tolerant monitoring of breathing through an acoustic sensor placed on the neck. The Radical-7's RRa measurement also provides an important visual indication of breathing through the displayed acoustic waveform.
The 2012 Radical-7 unleashes the power of rainbow® to help empower clinicians to improve patient outcomes and reduce the cost of care in multiple ways, such as reducing blood transfusion-related costs and detecting bleeding earlier with SpHb, optimizing fluid management with PVI, and enabling reliable detection of respiratory depression with RRa.
Joe Kiani, Masimo CEO and Chairman of the Board, stated: "We are very proud to introduce the 2012 Radical-7 to the anesthesia community at the World Congress of Anaesthesiologists. In 2000, we 'wowed' the healthcare industry with the original Radical. We believe the industry will once again be 'wowed' by the new clinician-centric Radical-7 and what it can do to improve the process of care and ultimately, patient care."
The 2012 Radical-7 can be seen at the World Congress of Anaesthesiologists at Exhibit 64-65. Masimo also will be hosting a sold-out industry symposium, "Improved Patient Outcomes and Decreased Cost of Care with Masimo rainbow® Technology," on Monday, March 26. In addition, Masimo will be hosting educational seminars in Exhibit 64-65 throughout the meeting.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Acquires Assets of Spire Semiconductor to Advance Innovation, Speed Development, and Reduce Costs
Irvine, California – March 12, 2012 – Masimo (NASDAQ: MASI), the inventor of Masimo Rainbow Pulse CO-Oximetry™, Masimo Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced it has acquired substantially all of the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies. Masimo Semiconductor Inc., a newly-formed, wholly-owned subsidiary of Masimo Corporation, will operate the business going forward. The acquisition gives Masimo an advanced ability to develop custom components, accelerate development cycles, and optimize future product costs.
Spire Corporation CEO and Chairman of the Board, Roger Little stated: "Spire Semiconductor has developed a superior intellectual property portfolio and advanced optoelectronic technology that is well-suited for Masimo's groundbreaking noninvasive patient monitoring technologies. For the past several years, Masimo has been one of Spire Semiconductor's largest customers. Masimo can now leverage Spire Semiconductor's strengths for an even greater impact on Masimo's industry leading innovations."
Joe Kiani, Masimo CEO and Chairman of the Board stated, "We believe that further advancements in noninvasive blood constituent monitoring technologies are unlikely using off-the-shelf optoelectronic components. Masimo Semiconductor will make the future possible and usher in a new era of technologic advancements for Masimo and ultimately our customers and patients."
Masimo Semiconductor, based in Hudson, N.H., specializes in wafer epitaxy, foundry services, and device fabrication for biomedical, telecommunications, consumer products and other markets. Masimo Semiconductor will continue to lead the industry in development of custom optoelectronic components, concentrator photovoltaic (CPV) cells, photocathodes, PIN diodes, Avalanche diodes, laser power converters, thermo photovoltaics and laser diodes.
About Spire Corporation
Spire Corporation is a diversified company serving the solar energy, biomedical, telecommunications and defense industries worldwide with innovative products and services based upon a common technology platform. For further details on the Company and its products, please visit www.spirecorp.com.
Contact
Spire Corporation
Roger G. Little
Chairman & CEO
(781) 275-6000
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Sana Kliniken AG Signs Agreement with Masimo, Giving One of Germany's Largest Private Hospital Networks Access to Masimo's Advanced Noninvasive Patient Monitoring Solutions
Deal Enables Sana to Move Toward a Standardized Oximetry Technology for Connected Hospitals
Irvine, California – March 6, 2012 – Sana Kliniken AG, a cooperative of 47 owned/partly owned and managed private hospitals as well as more than 450 purchasing cooperation partners throughout Germany, and Masimo (NASDAQ: MASI) jointly announce a pulse oximetry supplier agreement that makes Masimo's full line of noninvasive patient monitoring solutions available on Sana Kliniken's medical equipment purchasing contract. Now, in addition to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry solutions, all hospitals owned, managed, and contracted with Sana Kliniken AG will also be able to access and purchase the Masimo rainbow® SET Pulse CO-Oximeters and sensors offering advanced noninvasive physiological measurements at significant contracted savings.
"As one of Germany's leading hospital networks of acute and specialized medical services, the strength of the care we provide relies on the high-quality clinical expertise and leading medical equipment solutions we employ," said Olaf Buhr, head of strategic purchasing at Sana Kliniken AG. "We believe that Masimo rainbow SET technology uniquely positions our affiliated hospitals to better care for today's patient populations and cost-effectively prepares them for the future challenges facing health care."
Effective January 1, 2012 through January 1, 2016, Sana Kliniken member hospitals can purchase Masimo standalone (Radical-7®, Rad-87®, Rad-8) and portable (Rad-5, Rad-57®, Pronto, Pronto-7™) rainbow SET Pulse CO-Oximeters on contract, along with ReSposable, single patient use/disposable, and reusable sensors for all categories of patients (from neonatal and infant/pediatric to adult).
Masimo rainbow SET Pulse CO-Oximetry™ is a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow SET provides real-time results that enable clinicians to more rapidly assess patients and detect and treat potentially life-threatening conditions earlier.
At the heart of the Masimo rainbow SET technology platform is Masimo SET® (Signal Extraction Technology), clinically proven in more than 100 independent studies to provide accurate and reliable SpO2 and pulse rate measurements, even under the most challenging conditions of patient motion and low peripheral perfusion. In doing so, Masimo SET has been shown to reduce false patient monitor alarms by over 95% and expand true alarm detection to over 97%—making it the leading Measure-Through Motion and Low Perfusion SpO2 solution incorporated into more than 100 multi-parameter monitors and 50 monitoring brands.
As an upgradeable oximetry technology platform enabling new device capabilities to be added through simple field-installed software upgrades, Masimo rainbow Pulse CO-Oximeters allow healthcare facilities to fight the rising costs of equipment obsolescence. Instead of costly device and hardware replacements, new noninvasive measurements and patient monitoring capabilities can be cost-effectively added to existing "rainbow-ready" Masimo oximeters via a quick and easy field-installed software upgrader tool.
Masimo Founder and CEO, Joe Kiani said, "Representing one of Germany's largest private hospital networks, we are pleased that Sana Kliniken has chosen to make Masimo's full line of Masimo SET and Masimo rainbow SET oximetry solutions available to all its member hospitals. This agreement will ensure that access to the most advanced noninvasive patient monitoring solutions is available for the medical care and treatment of patients throughout Germany."
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: http://www.masimo.com/cpub/clinicals.htm.
About Sana Kliniken AG
Sana Kliniken AG consists of 47 owned hospitals and over 450 purchasing cooperation partners caring for more 1.334.000 patients. In addition to the core business of acute medicine, Sana includes specialist clinics is in the promising indications of heart/cardiovascular, orthopedics, and Neurology, as well as clinics and elder care facilities. This network of integrated health care is different from other private competitors as there are 31 private health insurers behind Sana. For more information, visit: www.sana.de
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Susanne Heintzmann
Sana Kliniken AG
Phone: 089 678204 334
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Present at 24th Annual ROTH Conference
IRVINE, Calif., February 27, 2012 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 24th Annual ROTH Conference at the Ritz Carlton Laguna Niguel in Dana Point, Calif. on Monday, March 12, 2012, at 2 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statement
This press release includes forward-looking statements based on current expectations about future events affecting us, all of which are subject to risks and uncertainties, difficult to predict and could cause our actual results to differ materially and adversely. These include statements about our technology, focus and prospects, among others. Risks and uncertainties include those related to our technology, competition and intellectual property protection, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

GE Healthcare Partners with Masimo to Incorporate
rainbow® SET Technology with GE Patient Monitors
Joint Offering Supports Efficient Clinical Decision-Making for Patients
Milwaukee, WI & Irvine, CA – February 23, 2012 – GE Healthcare andMasimo (NASDAQ: MASI) jointly announced a long-term agreement under which GE will incorporate Masimo rainbow® SET technology into many of GE Healthcare's patient monitoring products.
"Combining Masimo's measurements advances with our innovative patient monitors enhances bedside clinical decision-making and ultimately, supports patient care," said Matthias Weber, General Manager of Monitoring Solutions at GE Healthcare. "Through our focused Monitoring Solutions division, GE Healthcare is committed to optimally collecting information from the patient's body, and transforming it into meaningful intelligence for caregivers. Masimo's suite of noninvasive and continuous rainbow® SET measurements will further enhance the point-of-care clinical intelligence benefits already offered in GE Healthcare's patient monitors."
Masimo rainbow® SET noninvasive and continuous measurements—including total hemoglobin (SpHb®) , oxygen content (SpOC™), carboxyhemoglobin (SpCO®) , methemoglobin (SpMet®) , and Pleth Variability Index (PVI®) , in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide additional physiological data at the point-of-care that can help clinicians assess patients and detect adverse conditions much earlier. These measurements help caregivers identify conditions such as low levels of blood oxygen, hemoglobin, and/or perfusion, as well as elevated levels of carbon monoxide and methemoglobin in the blood, abnormal pulse rates, and fluid responsiveness. With rainbow® SET, the ability to noninvasively detect and continuously monitor changes quickly and easily may help save lives and improve patient outcomes.
According to Masimo President of Worldwide OEM Business & Corporate Development, Rick Fishel, "GE Healthcare's patient monitoring lines have a long history of innovative clinical measurements and system solutions that enhance patient care decisions. We are excited to announce this expansion of our partnership with GE Healthcare and are proud to work with them to incorporate rainbow SET measurements in their monitoring solutions to continue this tradition for the benefit of customers and the patients they serve."
About GE Healthcare
GE Healthcare provides transformational medical technologies and services that are shaping a new age of patient care. Our broad expertise in medical imaging and information technologies, medical diagnostics, patient monitoring systems, drug discovery, biopharmaceutical manufacturing technologies, performance improvement and performance solutions services help our customers to deliver better care to more people around the world at a lower cost. In addition, we partner with healthcare leaders, striving to leverage the global policy change necessary to implement a successful shift to sustainable healthcare systems. Our "healthymagination" vision for the future invites the world to join us on our journey as we continuously develop innovations focused on reducing costs, increasing access and improving quality around the world. Headquartered in the United Kingdom, GE Healthcare is a unit of General Electric Company (NYSE: GE). Worldwide, GE Healthcare employees are committed to serving healthcare professionals and their patients in more than 100 countries. For more information about GE Healthcare, visit our website at www.gehealthcare.com. For our latest news, please visit http://newsroom.gehealthcare.com
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results, risks related to our belief that the integration of Masimo rainbow SET into GE patient monitoring devices will help improve patient care and save lives, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Sheree Aronson
Masimo Corporation
Phone: (949) 297-7043
Email: [email protected]
Annette Busateri
GE Healthcare
Phone: (262) 442-0966
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Announces "Better Care" Program— A Hospital Risk-Share Program for Blood Transfusion Related Cost Reduction with SpHb®, and $30 SpHb Sensor Price
Better Care Program Enables Select Hospitals to Adopt SpHb Hospital-Wide to Improve Patient Outcomes with Guaranteed Savings
Irvine, California – February 14, 2012 – Masimo (NASDAQ: MASI) today announced that in line with its mission to "Improve Patient Outcomes and Reduce the Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®," and its confidence that continuous noninvasive hemoglobin monitoring (SpHb®) will further the company's stated mission, Masimo is introducing a hospital-wide risk-share program for Blood Transfusion Related Cost Reduction (BTR-CR, pronounced "Better Care"). The Better Care Program guarantees blood transfusion related cost reductions will exceed the incremental price of SpHb sensors.
Joe Kiani, Founder and CEO of Masimo stated, "We understand that hospitals are doing their best to deliver quality care in an unprecedented time of reduced census, cost containment pressure, and increasing expense. Through our customers' and independent researchers' experience with SpHb, we are confident that SpHb will not only reduce mortality and morbidity, but will reduce cost dramatically. The Better Care Program allows a select number of hospitals to implement SpHb monitoring hospital-wide with the guaranteed protection that their costs will not increase. We hope the Better Care Program will not only provide improved patient care and dramatically lower costs, but will also represent a new model for other medical technology companies to help hospitals achieve their goal of better care, with lower cost guaranteed!"
Masimo's Better Care Risk-Share Program—Guarantees Hospital Savings from SpHb
Reducing cost of care is a central focus in every hospital today. Red blood cell transfusions and their related costs are one of the largest cost centers in every hospital, often 10 times the annual cost of SpO2 sensor purchases. Transfusions can save lives, but the evidence is clear that they also dramatically increase mortality and morbidity risk and cost. These facts underscore the need to transfuse only when necessary. Hemoglobin concentration is a primary indicator of the need for transfusion and invasive blood draws for laboratory hemoglobin determination are among the most common tests performed in hospitals, but they can only offer intermittent and often delayed results. Masimo SpHb has been shown to help clinicians reduce blood transfusions during surgery, as well as provide an early indication of hemoglobin drops in medical and surgical intensive care, obstetric, and general-floor patients—allowing lifesaving interventions. Results of a randomized controlled trial conducted by researchers at Massachusetts General Hospital and Harvard Medical School showed that Masimo SpHb helped anesthesiologists reduce the frequency of blood transfusion by 90% (from 0.1 to 0.01 units per patient) in 327 patients undergoing orthopedic surgery.1
In keeping with Masimo's mission to improve patient outcomes and reduce the cost of care, Masimo is proud to introduce its risk-share program for Blood Transfusion Related Cost Reduction. Masimo guarantees that when a hospital replaces its standard SpO2 adhesive sensors with Masimo rainbow® ReSposable SpHb Sensors and begins monitoring noninvasive hemoglobin (SpHb), pleth variability index (PVI®), and methemoglobin (SpMet®), in addition to Masimo SET SpO2, pulse rate, and perfusion index (PI), the incremental price paid for the rainbow sensors will be exceeded by a reduction in blood transfusion related costs.
If the documented blood transfusion-related costs in a hospital do not exceed the incremental price paid for Masimo rainbow® ReSposable Sensors beyond standard SpO2 sensor pricing, Masimo will provide a refund for the difference between the incremental price paid and the reduction in blood transfusion related costs (terms and conditions apply). The Better Care Program is available to the first 100 hospitals worldwide that qualify for the program. Interested hospitals should contact their Masimo sales representative or go online for further details and conditions at: www.masimo.com/bettercare.2
"Less than five percent of hospital costs are associated with the use of medical devices and disposables," stated Jon Coleman, President of Worldwide Sales, Marketing and Clinical Research at Masimo. "In contrast, blood is one of the largest cost centers in the hospital. Masimo is so confident that SpHb monitoring will result in a blood transfusion related cost reduction that we are willing to guarantee it with our new Better Care Risk-Share Program. We look forward to partnering with hospitals around the world to improve patient outcomes and decrease the cost of care with SpHb and our other rainbow® monitoring capabilities."
We believe hospitals can expect even greater savings beyond blood transfusion related costs by using SpHb and other rainbow® measurements to detect bleeding sooner and enable lifesaving interventions, reduce the number of blood draws and incidents of hospital-acquired anemia, optimize fluid management with PVI, and decrease the risk of dangerous methemoglobinemia with SpMet. In addition, we believe hospitals will also receive clinical and financial benefits from Masimo SET pulse oximetry, including reduced retinopathy of prematurity (ROP) in very low birth weight neonates, decreased sensor utilization, decreased arterial blood gas measurements, faster oxygen weaning time, and earlier detection of patient distress that helps to improve outcomes and lower the length of stay for post-surgical patients. The rainbow® ReSposable Sensor also provides the industry's best green solution with a dramatically lower carbon footprint.3
New Design Leads to $30 rainbow® SpHb ReSposable™ Sensor Price—Making SpHb Use Broadly Available
Thanks to recent enhancements to the rainbow® ReSposable design that increase the number of patient applications possible with the Reusable Optical Sensor (ROS™) component of the ReSposable System to 20 or more patients (from five previously), Masimo's internal cost savings can be extended to hospitals wishing to adopt SpHb more widely. The SpHb ReSposable Sensor System is now priced at just $30 USD per patient (based on reasonable committed sensor usage), which represents up to a 50% price reduction over previous pricing. Given that the SpHb ReSposable Sensor also provides Masimo SET® SpO2, pulse rate, perfusion index (PI), methemoglobin (SpMet), and pleth variability index (PVI) measurements, the new sensor pricing makes it possible for SpHb to become a new standard of care for all patients throughout the continuum of care.
1 Ehrenfeld J., Henneman J. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." American Society of Anesthesiologists, 2010 Annual Conference, October 18, 2010. http://www.asaabstracts.com/strands/asaabstracts/search.htm;jsessionid=BD0FC2767124489F77146E7C70A95EC5.
2 This is not an offer. Participating in the guarantee requires a contract with agreed-upon terms and conditions.
3 Based on carbon footprint calculations validated by Carbonfund.org in November 2011.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that SpHb enables hospitals to achieve significant patient care and financial impacts, risks related to our Better Care Risk Share Program and belief that the incremental price paid for the rainbow sensors will be exceeded by a reduction in blood transfusion related costs in every hospital, risks related to our belief that SpHb ReSposable sensor enhancements will deliver a 20:1 use ratio in all circumstances and applications, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Reports Fourth Quarter and Full Year 2011 Financial Results; Provides 2012 Financial Guidance
Q4 2011 Highlights (compared to Q4 2010):
- Total revenue, including royalties, rose 6% to $112.3 million.
- Product revenue rose 12% to $104.7 million.
- Shipped 34,400 Masimo SET® and Masimo rainbow® SET units.
- Masimo rainbow revenue rose 16% to $9.8 million.
- GAAP EPS was $0.23 versus $0.26 in the year-ago period. Excluding one-time items in Q4 2010, GAAP EPS declined 21% in Q4 2011, compared to adjusted EPS of $0.29 in Q4 2010.
Full Year 2011 Highlights (compared to 2010):
- Total revenue, including royalties, rose 8% to $439.0 million.
- Product revenue rose 14% to $406.5 million.
- Shipped 148,200 Masimo SET® and Masimo rainbow® SET units, increasing worldwide installed base by 15%.
- Masimo rainbow revenue rose 4% to $34.1 million. Excluding the 2010 U.S. military order, rainbow revenue rose 18%.
- GAAP EPS was $1.05 versus $1.21 in 2010, which included a one-time net gain of $0.18. Excluding one-time items in 2010, GAAP EPS rose 2% in 2011 from $ 1.03.
Irvine, California, February 14, 2012 – Masimo (NASDAQ: MASI) today announced its financial results for the fourth quarter and year ended December 31, 2011.
Masimo's total fourth quarter revenue, including royalties, rose 6% to $112.3 million, compared to $105.6 million for the fourth quarter of 2010. Fourth quarter 2011 product revenue rose 12% to $104.7 million, compared to $93.8 million for the fourth quarter of 2010. For the fourth quarter of 2011, the company's worldwide end-user business grew 17% and represented 86% of product revenue. OEM sales, which represented the remaining 14% of product revenue, declined 12% in the fourth quarter of 2011, compared to the same period in 2010. Revenue from Masimo rainbow product sales rose 16% to $9.8 million in the fourth quarter, compared to $8.4 million for the fourth quarter of 2010.
Net income for the fourth quarter was $13.8 million, or $0.23 per diluted share, compared to reported net income of $16.1 million, or $0.26 per diluted share, in the fourth quarter of 2010. Excluding one-time expenses in the year-ago period, Masimo's adjusted net income and adjusted earnings per share for the fourth quarter of 2010 were $17.5 million and $0.29, respectively.
For 2011, Masimo's total revenue rose 8% to $439.0 million, compared to $405.4 million in 2010. The company's product revenue rose 14% to $406.5 million, compared to $356.4 million in 2010. Revenue from Masimo rainbow products rose 4% to $34.1 million, compared to $32.9 million in 2010. Excluding the $4.0 million U.S. military order in 2010 that did not repeat in 2011, rainbow revenue grew by18%. Royalty revenue declined from $49.0 million in 2010 to $32.5 million in 2011.
Masimo's net income for 2011 was $63.7 million, or $1.05 per diluted share, compared to 2010 net income of $73.5 million, or $1.21 per diluted share, which included $0.18 from the net of a one-time gain related to $30.8 million in antitrust proceeds from Covidien and offset by $14.7 million in one-time expenses. Excluding one-time items in 2010, Masimo's 2010 adjusted net income and adjusted earnings per share were $62.5 million and $1.03, respectively.
During the fourth quarter, the company shipped approximately 34,400 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, down 18% compared to approximately 41,800 in the same prior-year period. For 2011, Masimo shipped approximately 148,200 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, down 2% compared to approximately 153,200 in 2010. Masimo estimates its worldwide installed base as of December 31, 2011 to be 979,000 units, up 15% from 855,000 units as of January 1, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo finished 2011 with a 12% rise in fourth quarter product revenue, including 17% growth in our direct business. Strong double-digit sales growth in our U.S. acute care channel and international business, as well as a 16% increase in rainbow revenue, drove our product revenue performance in the quarter. We also grew our worldwide installed base by 15%, expanding our global reach and demonstrating the continuing demand for our superior Masimo SET pulse oximetry and rainbow SET Pulse CO-Oximetry technology."
As of December 31, 2011, cash and cash equivalents were $129.9 million, compared to $88.3 million as of January 1, 2011. During the fourth quarter, the company used approximately $36 million in cash to purchase approximately 1.8 million shares of its common stock under a stock repurchase program authorized by the Board of Directors in mid-2011.
2012 Financial Guidance
Masimo expects fiscal 2012 total revenue to be approximately $483 million, including product revenue of $455 million and royalty revenue of $28 million. Included within the 2012 product revenue guidance is a rainbow revenue expectation of $45 million. The company expects fiscal 2012 GAAP earnings per share to be $1.20. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 42982286. After the live webcast, the call will be available on Masimo's website through March 14, 2012. In addition, a telephonic replay of the call will be available through February 28, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 42982286
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; demand for our technologies; and expectations for total revenue, royalty revenue and product revenue, including rainbow revenue, and GAAP earnings per share, for the full fiscal year 2012. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor
Relations, Masimo Corporation
(949) 297-7043
[email protected]
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


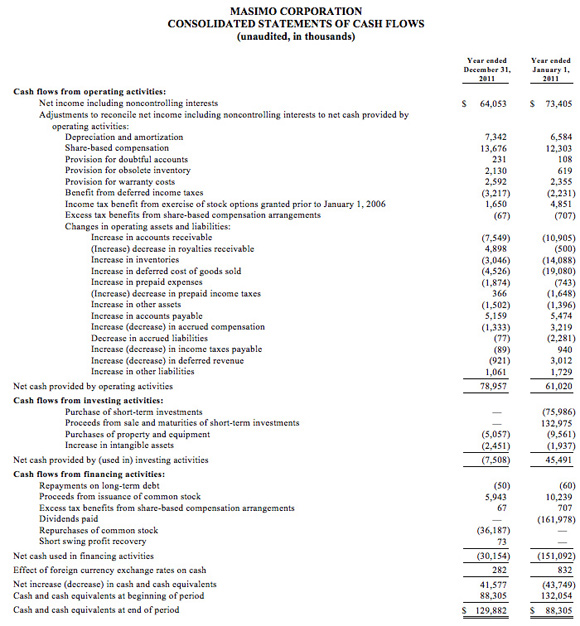


Masimo to Report Fourth Quarter and Full Year 2011 Financial Results After Market Close on February 14, 2012
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 26, 2012 -- Masimo (NASDAQ: MASI) announced today that it will release fourth quarter and full year financial results for the period ended December 31, 2011, after the market closes on February 14, 2012. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 42982286. After the live webcast, the call will be available on Masimo's website through March 14, 2012. In addition, a telephonic replay of the call will be available through February 28, 2012. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 42982286.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
[email protected]
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces FDA Clearance and Full Market Release of the New Pronto-7™ for Noninvasive Total Hemoglobin Spot-Check Measurement, Along with SpO2, Pulse Rate, and Perfusion Index
Irvine, California – January 9, 2012 – Masimo (NASDAQ: MASI) today announced FDA 510(k) clearance and full market commercial launch of the Masimo Pronto-7®—a palm-sized handheld device designed for quick and easy noninvasive spot-checking of total hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index.
Hailed as a "potential game-changing technology," Dr. Andrew J. Schuman—adjunct assistant professor of pediatrics at Dartmouth Medical School, contributing editor for Contemporary Pediatrics, and a practicing pediatrician in New Hampshire, who reviewed the device prior to FDA clearance—says that the new Pronto-7 is so innovative it has the "potential for significantly improving pediatric practice."
"The Pronto-7 is a potentially game-changing device because it can help healthcare providers expedite the diagnosis and treatment of anemia in children and adolescents," continued Dr. Schuman. Not only does it represent an exciting medical advancement in point-of-care testing, but it also provides pediatricians with a high-tech alternative to painful venipuncture or finger stick blood sampling that, until now, has been the only way to measure hemoglobin levels in our young patients. Parents and pediatricians will also appreciate our new ability to screen without the scream! Most would agree that the best preventative healthcare is provided both quietly as well as painlessly."
With FDA 510(k) clearance and full commercial availability of the new Pronto-7 device, which includes expanded sensor size options to accommodate a wider range of finger sizes and the addition of a Max Sensitivity Mode, clinicians throughout the United States will be able to quickly and conveniently measure total hemoglobin, SpO2, pulse rate, and perfusion index— without removing a drop of blood.
 Masimo Pronto-7 has already received several notable industry accolades, including the 2011 GOLD Medical Design Excellence Award, 2011 TechAmerica High-Tech Innovation Award, and the 2011 iF Product Design Award. And in2010, the Pronto-7 was named to the World Health Organization's (WHO) List of Innovative Medical Technologies that Address Global Health Concerns and Needs.
Masimo Pronto-7 has already received several notable industry accolades, including the 2011 GOLD Medical Design Excellence Award, 2011 TechAmerica High-Tech Innovation Award, and the 2011 iF Product Design Award. And in2010, the Pronto-7 was named to the World Health Organization's (WHO) List of Innovative Medical Technologies that Address Global Health Concerns and Needs.
Hemoglobin is one of the most commonly ordered tests in both hospital and non-hospital settings because it is critical to assessing blood loss. However, current testing requires a painful needle stick for the patient, time-consuming blood draws for the clinician, and typically provides delayed results. The Pronto-7 offers a breakthrough solution for measuring hemoglobin in less than one minute—without the needles, risk of blood contamination, hazardous medical waste, laboratory analysis, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, pulse rate, and perfusion index, into any clinician's hands in various clinical settings, including physician offices, hospitals, and clinics. Because of the device's embedded 802.11 b/g and Bluetooth communication capability, wireless printing or emailing of test results is enabled and future upgrades will allow for wireless transmission to electronic health record (EHR) systems.
Thomas A. Murray, III, MD, an obstetrician/gynecologist in Salem, Massachusetts, says: "As one of the first users of the prototype Pronto-7, I was impressed with its ease of use right from the start. In a general ob/gyn office, anemia is a common disorder and the Pronto-7 allows me to accurately measure my patients' hemoglobin levels noninvasively, conveniently, and quickly—right here on-the-spot in my office. My patients used to cringe at the idea of a finger stick and love that Pronto-7 is noninvasive. It is also much safer for my staff as there is no possibility of a needle stick injury or blood exposure. I have found the Pronto-7 to be very useful in my practice."
Masimo is the first company to commercially introduce continuous and spot-check noninvasive total hemoglobin devices. Prior to the introduction of the Pronto-7, Masimo received U.S. FDA 510(k) and CE mark clearance and first introduced the ability to noninvasively and continuously measure total hemoglobin (SpHb) in 2008 using its Radical-7 bedside Pulse CO-Oximeter. In 2009, Masimo launched Pronto®—its first handheld noninvasive spot-check device for point-of-care hemoglobin, SpO2, and pulse rate testing—worldwide. And in 2010, Medicare reimbursement was added for transcutaneous hemoglobin measurement in the United States.
Masimo Founder and CEO, Joe Kiani, stated: "Full market release of Pronto-7 will enable clinicians to better care for and assess their patients. We agree with the physicians and industry experts who call the Pronto-7 a game-changing technology. And we are proud to deliver this medical first—completely noninvasive total hemoglobin, SpO2, pulse rate, and perfusion index measurements available in the palm of your hand—which offers healthcare providers around the world the opportunity to transform the way and amount of time it takes to deliver the right care, at the right time to patients."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the new Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.











